Understand the Problem
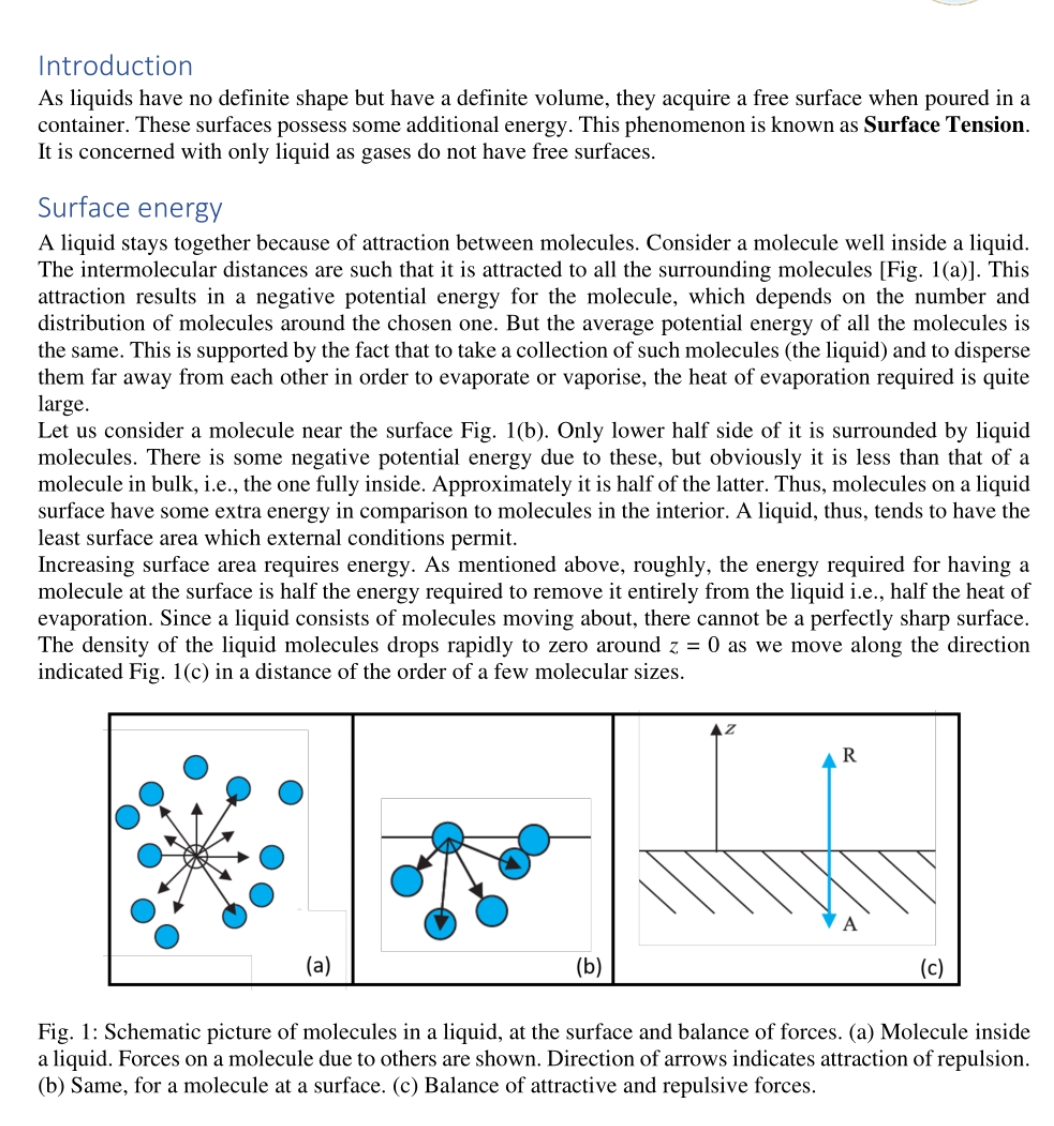
The text discusses surface tension and surface energy in liquids, explaining the molecular interactions that cause a liquid to maintain its shape and the energy dynamics associated with surface molecules compared to those in the bulk.
Answer
Surface tension is the tendency of liquid surfaces to shrink to the minimum surface area due to molecular attraction.
Surface tension is a phenomenon where the surface of a liquid acts as if it is covered by a stretched elastic membrane due to the attraction between molecules at the surface.
Answer for screen readers
Surface tension is a phenomenon where the surface of a liquid acts as if it is covered by a stretched elastic membrane due to the attraction between molecules at the surface.
More Information
Surface tension arises because the molecules at the surface of a liquid are attracted to each other, creating a 'tension' on the surface.
Tips
A common mistake is not recognizing that only liquids exhibit surface tension because gas molecules don't possess free surfaces where these interactions can occur.
AI-generated content may contain errors. Please verify critical information