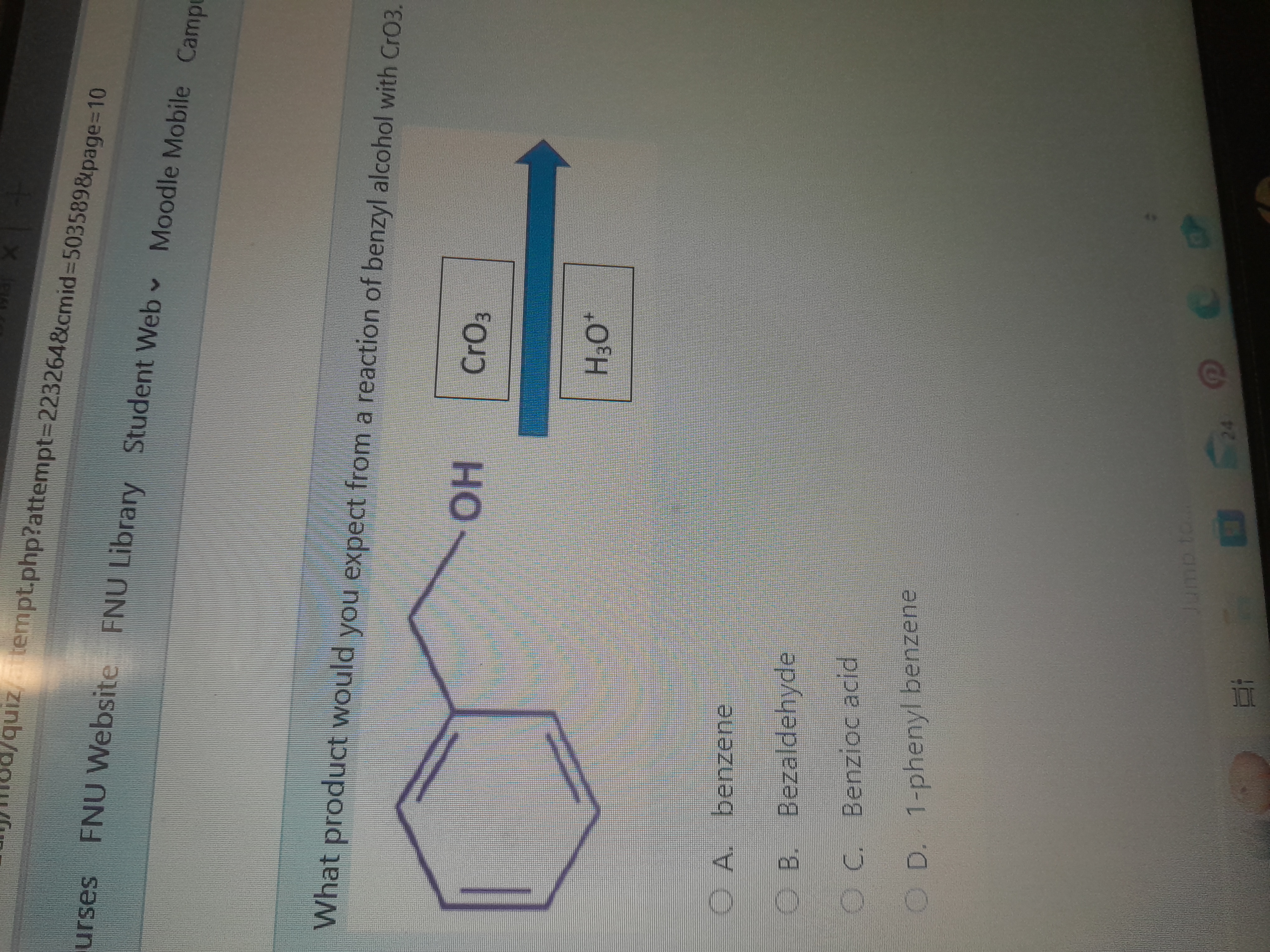
What product would you expect from a reaction of benzyl alcohol with CrO3?
Understand the Problem
The question is asking what product is formed when benzyl alcohol reacts with chromium trioxide (CrO3) under acidic conditions. This involves understanding the oxidation reaction that occurs in organic chemistry.
Answer
Benzoic acid
The final answer is benzoic acid.
Answer for screen readers
The final answer is benzoic acid.
More Information
Benzyl alcohol is a primary alcohol and is oxidized by CrO3 to form benzoic acid, a common mechanism where primary alcohols are oxidized to carboxylic acids.
Tips
A common mistake is assuming benzyl alcohol will form an aldehyde; however, CrO3 is a strong oxidizer that converts primary alcohols to carboxylic acids.
Sources
- 17.7: Oxidation of Alcohols - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information