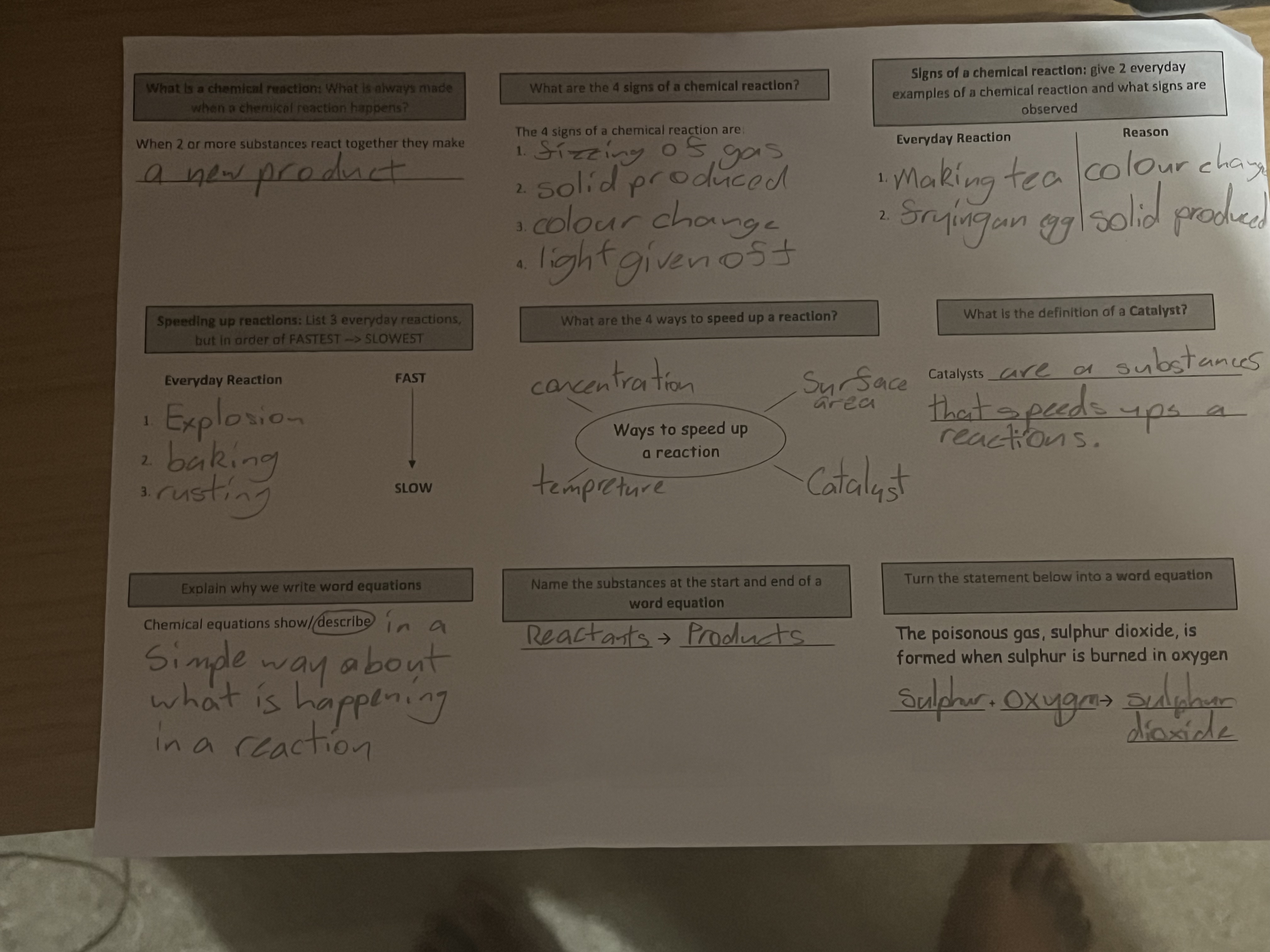
What is a chemical reaction? What is always made when a chemical reaction happens? List 2 everyday examples of a chemical reaction and what signs are observed. How can you speed up... What is a chemical reaction? What is always made when a chemical reaction happens? List 2 everyday examples of a chemical reaction and what signs are observed. How can you speed up a reaction? What is the definition of a catalyst? Explain why we write word equations. Name the substances at the start and end of a word equation. Turn the statement below into a word equation: The poisonous gas, sulphur dioxide, is formed when sulphur is burned in oxygen.
Understand the Problem
The question pertains to chemical reactions, including definitions, examples, and chemical equations. It asks about what happens during chemical reactions, how to speed them up, signs of chemical reactions, and the components involved in word equations.
Answer
A chemical reaction makes a new product. Examples: making tea, frying an egg. Speed up with concentration, temperature, surface area, or a catalyst. A catalyst speeds up reactions without being consumed. Word equations are simple descriptions; use reactants and products. Equation: Sulfur + Oxygen → Sulfur dioxide.
The final answer is:
- A chemical reaction leads to the formation of a new product.
- Everyday examples: making tea (color change), frying an egg (solid produced).
- Speed up reactions with concentration, temperature, surface area, or a catalyst.
- A catalyst increases reaction rate without being consumed.
- Word equations describe reactions simply.
- Reactants and products are at the start and end of a word equation.
- Word equation for given statement: Sulfur + Oxygen → Sulfur dioxide.
Answer for screen readers
The final answer is:
- A chemical reaction leads to the formation of a new product.
- Everyday examples: making tea (color change), frying an egg (solid produced).
- Speed up reactions with concentration, temperature, surface area, or a catalyst.
- A catalyst increases reaction rate without being consumed.
- Word equations describe reactions simply.
- Reactants and products are at the start and end of a word equation.
- Word equation for given statement: Sulfur + Oxygen → Sulfur dioxide.
More Information
Chemical reactions commonly lead to color changes or formations of new substances such as gases or solids. Catalysts are important in increasing the efficiency of many industrial and biological processes.
Tips
A common mistake is confusing a catalyst with a reactant or product, as it doesn't get consumed in the reaction.
Sources
- Examples of Chemical Reactions in Everyday Life - ThoughtCo - thoughtco.com
- Chemical reaction - Wikipedia - en.wikipedia.org
- DOE Explains...Catalysts - Department of Energy - energy.gov
AI-generated content may contain errors. Please verify critical information