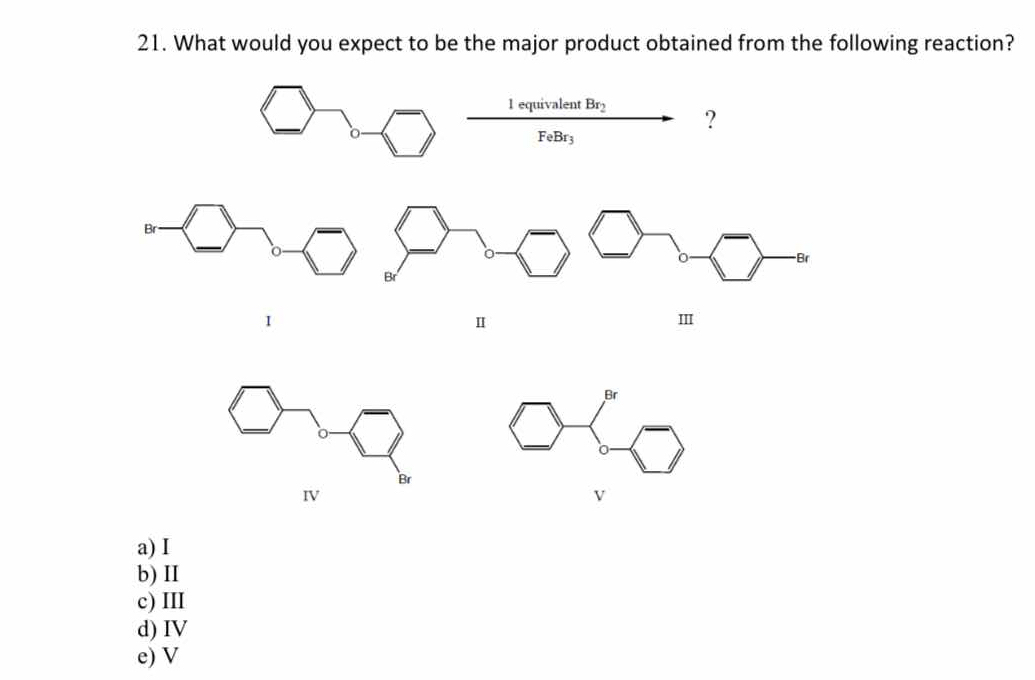
What would you expect to be the major product obtained from the following reaction involving 1 equivalent of Br2 and FeBr3?
Understand the Problem
The question is asking which compound (I, II, III, IV, or V) would be the major product resulting from a reaction involving bromine (Br2) in the presence of FeBr3. This is a typical question in organic chemistry focusing on electrophilic aromatic substitution.
Answer
IV
The major product is IV.
Answer for screen readers
The major product is IV.
More Information
In this reaction, FeBr3 acts as a Lewis acid and facilitates electrophilic aromatic substitution (bromination). The methoxy group is an ortho/para director, so bromination occurs at the para position relative to the methoxy group, resulting in product IV.
Tips
Ensure identification of directing groups and their effects on substitution patterns.
Sources
AI-generated content may contain errors. Please verify critical information