What are the two main conformations of ethane, and how do they differ?
Understand the Problem
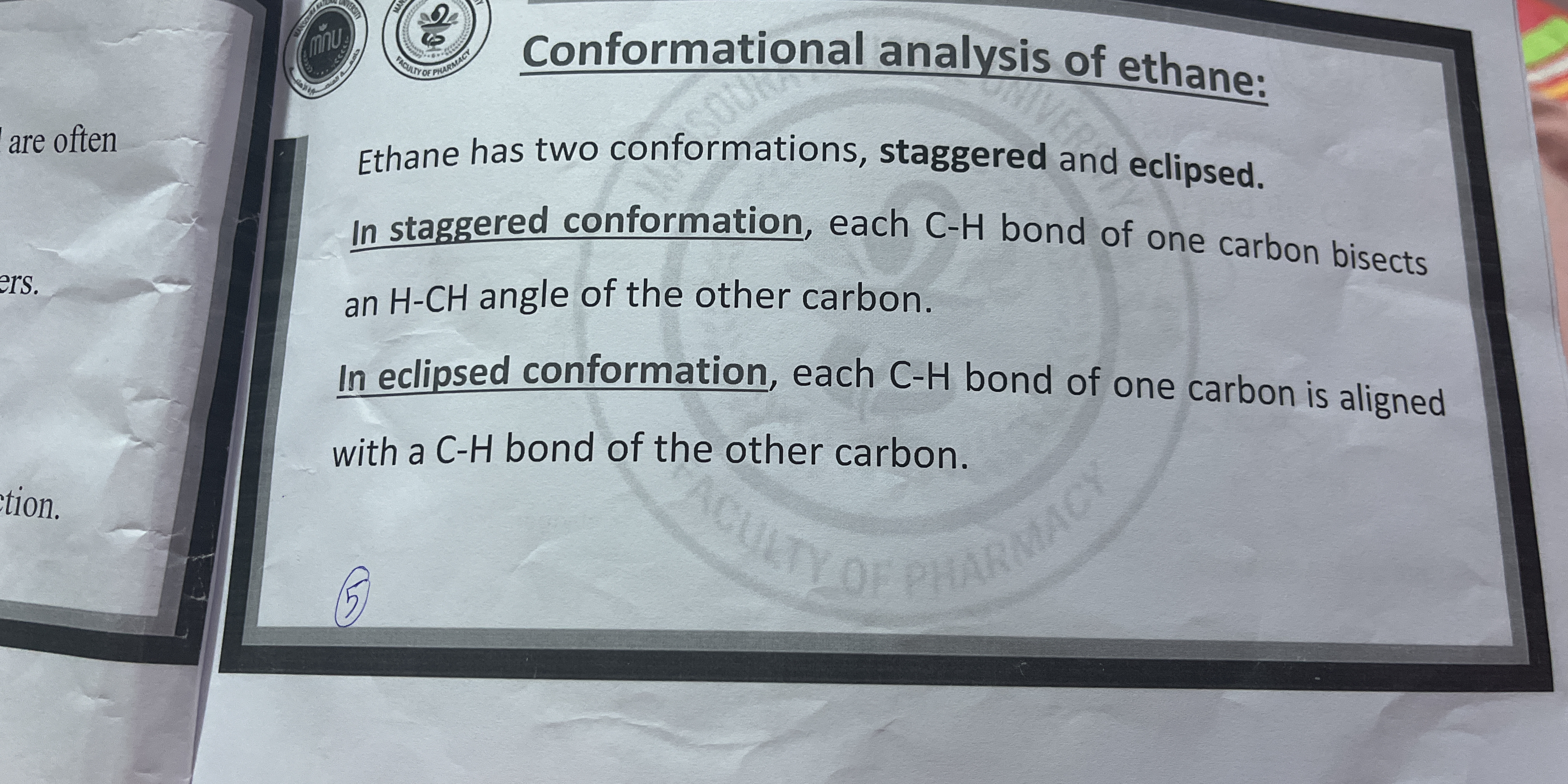
The question is describing the conformational analysis of ethane, focusing on its two main conformations: staggered and eclipsed. The text explains the differences in orientation of the C-H bonds in each conformation.
Answer
Ethane's conformations: staggered (more stable) and eclipsed (less stable).
The two main conformations of ethane are staggered and eclipsed. Staggered conformation is more stable with lower energy due to better spacing between C-H bonds, while eclipsed conformation is less stable with higher energy due to close alignment of C-H bonds.
Answer for screen readers
The two main conformations of ethane are staggered and eclipsed. Staggered conformation is more stable with lower energy due to better spacing between C-H bonds, while eclipsed conformation is less stable with higher energy due to close alignment of C-H bonds.
More Information
Staggered conformation occurs about 99% of the time due to its stability, while eclipsed occurs only about 1% of the time due to its higher energy level.
Tips
Common mistakes include confusing the stability levels of the conformations or forgetting to utilize Newman projections to understand spatial arrangements.
Sources
- Conformations of Ethane - Chemistry LibreTexts - chem.libretexts.org
- Staggered vs Eclipsed Conformations of Ethane - masterorganicchemistry.com
- Conformational Analysis of Ethane & Butane - sas.upenn.edu
AI-generated content may contain errors. Please verify critical information