How many moles of oxygen are needed to react with 23.8 moles of aluminum?
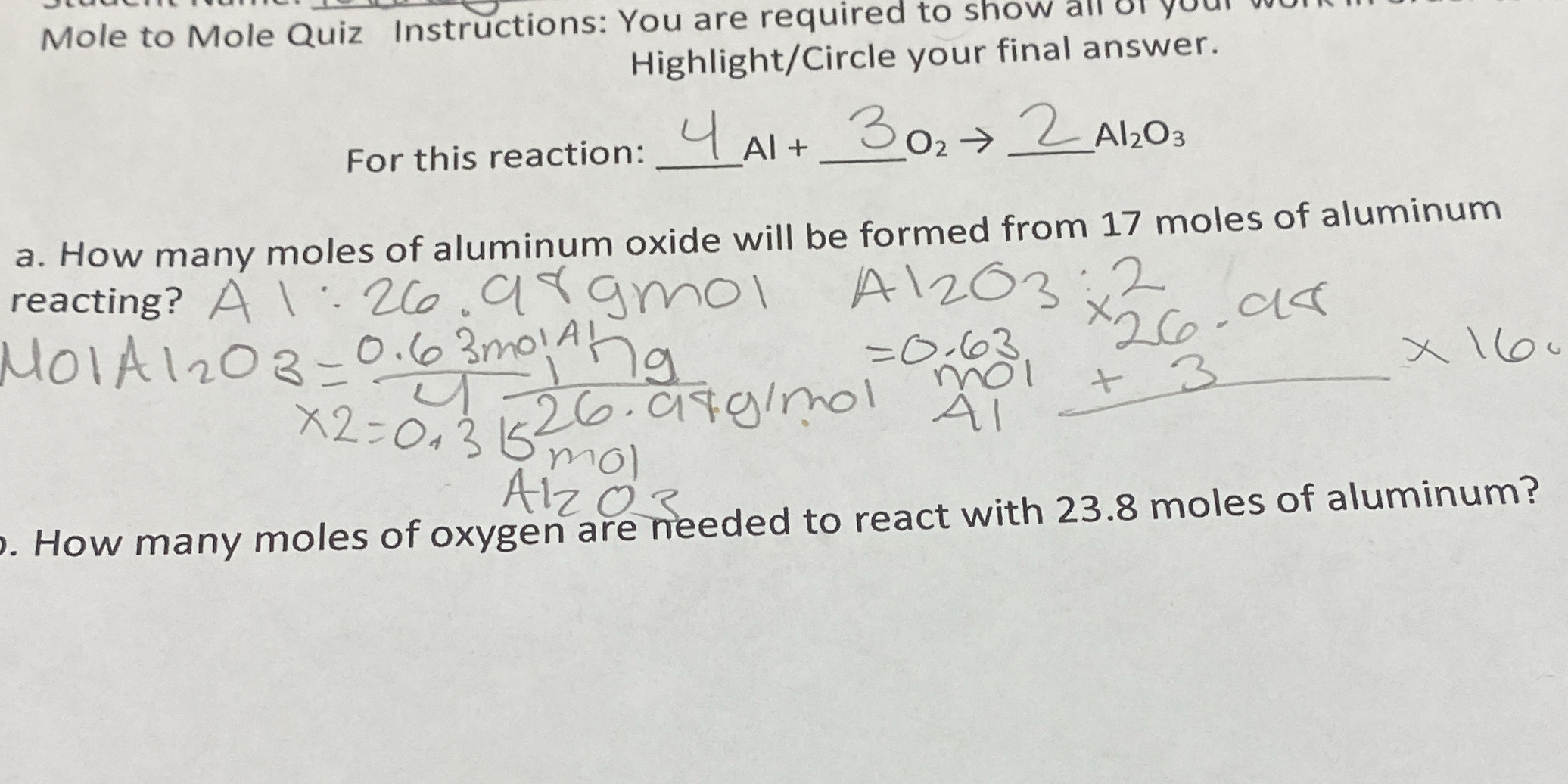
Understand the Problem
The question is asking how many moles of oxygen are required to react with 23.8 moles of aluminum, using stoichiometric principles from the balanced chemical reaction provided.
Answer
The moles of oxygen needed to react with 23.8 moles of aluminum are $17.85$.
Answer for screen readers
The number of moles of oxygen needed is $17.85$ moles.
Steps to Solve
- Identify the Reaction Stoichiometry
The balanced chemical equation is:
$$ 4 \text{Al} + 3 \text{O}_2 \rightarrow 2 \text{Al}_2\text{O}_3 $$
From this equation, we see that 4 moles of aluminum react with 3 moles of oxygen.
- Establish the Ratio
Set up the ratio of aluminum to oxygen from the balanced equation:
$$ \frac{3 \text{ moles O}_2}{4 \text{ moles Al}} $$
- Calculate Moles of Oxygen
Use the ratio to find out how many moles of oxygen are needed for 23.8 moles of aluminum:
$$ \text{Moles of O}_2 = 23.8 \text{ moles Al} \times \frac{3 \text{ moles O}_2}{4 \text{ moles Al}} $$
- Perform the Calculation
Now, calculate the moles of oxygen needed:
$$ \text{Moles of O}_2 = 23.8 \times \frac{3}{4} = 23.8 \times 0.75 = 17.85 $$
The number of moles of oxygen needed is $17.85$ moles.
More Information
In the reaction of aluminum with oxygen, the specific stoichiometric ratio is critical for determining the required amounts of reactants. The ratio indicates that for every 4 moles of aluminum, 3 moles of oxygen are consumed.
Tips
- Misinterpreting the Stoichiometry: Ensure that you correctly understand the ratio from the balanced equation. Remember that in stoichiometry, the coefficients represent the moles of each substance.
- Calculation Errors: Always double-check your arithmetic when multiplying or dividing. Small mistakes can lead to incorrect answers.
AI-generated content may contain errors. Please verify critical information