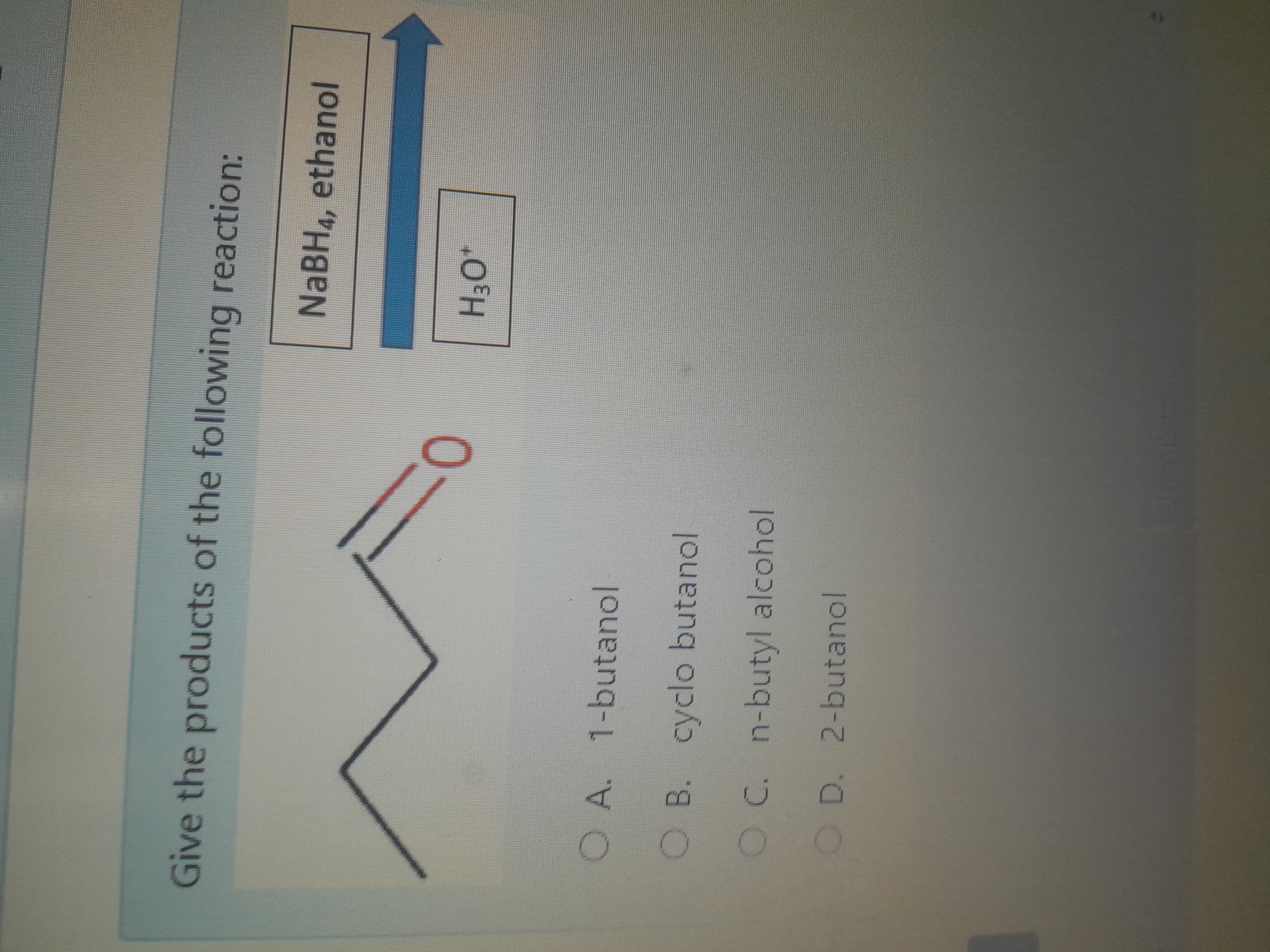
Give the products of the following reaction: NaBH4, ethanol, H3O+
Understand the Problem
The question is asking for the identification of the products resulting from a reaction involving a specific organic compound, sodium borohydride (NaBH4) in ethanol, and acid protonation. The aim is to determine the correct alcohol product formed after the reaction.
Answer
2-butanol
The final product is 2-butanol.
Answer for screen readers
The final product is 2-butanol.
More Information
Sodium borohydride (NaBH4) is a mild reducing agent commonly used in organic chemistry to reduce ketones and aldehydes to alcohols.
Tips
Ensure the correct structure of the starting compound is identified, as the specific alcohol formed depends on the position of the carbonyl group.
Sources
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones - masterorganicchemistry.com
- Reductions using NaBH4, LiAlH4 - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information