Determine the type of geometrical isomerism of the following compounds:
Understand the Problem
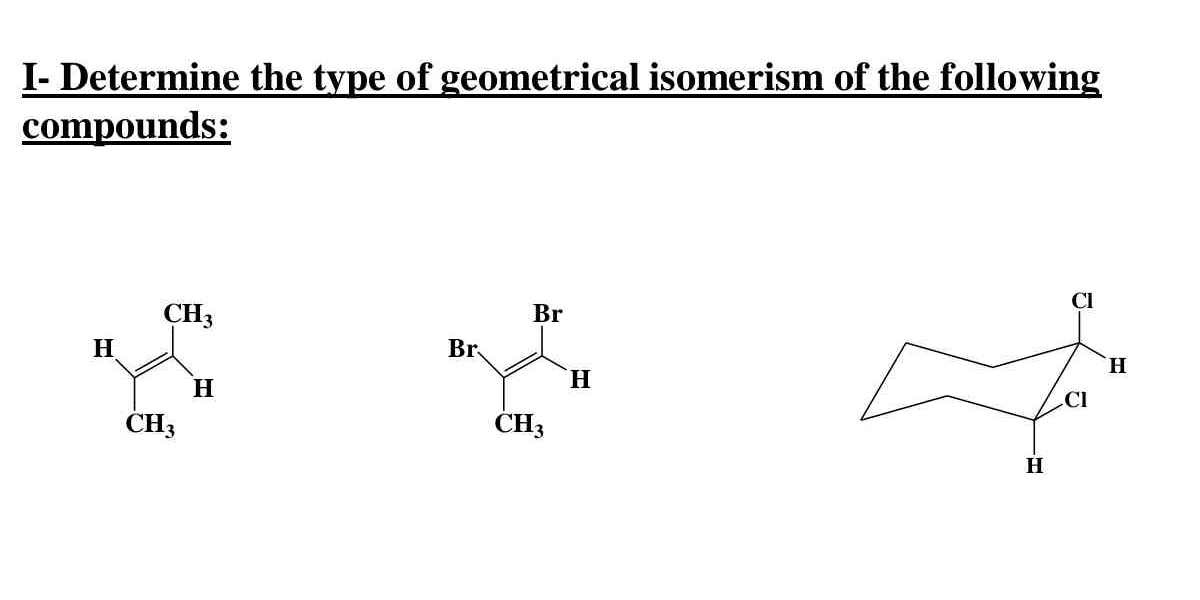
The question is asking to identify the types of geometrical isomerism present in the given chemical compounds. This will involve analyzing their structures and determining whether they exhibit cis/trans isomerism or other forms of stereoisomerism.
Answer
No isomerism; Cis; Trans
The first compound shows no geometrical isomerism. The second compound exhibits cis isomerism. The third compound shows trans isomerism.
Answer for screen readers
The first compound shows no geometrical isomerism. The second compound exhibits cis isomerism. The third compound shows trans isomerism.
More Information
Geometric isomerism occurs due to restricted rotation, often around double bonds or in rings. Cis-trans uses relative position of similar atoms/groups. Cyclohexane derivatives consider 3D chair conformation for isomerism.
Tips
Overlooking the similarity needed for cis-trans identification or the axial-equatorial positioning in cyclohexanes can lead to misidentification.
AI-generated content may contain errors. Please verify critical information