Podcast
Questions and Answers
What defines functional groups in biological molecules?
What defines functional groups in biological molecules?
What is the process called when two glucose molecules bond together to form maltose?
What is the process called when two glucose molecules bond together to form maltose?
What is the scientific term for the breakdown of a polymer into its constituent monomers?
What is the scientific term for the breakdown of a polymer into its constituent monomers?
Which of the following statements is true regarding glucose and fructose?
Which of the following statements is true regarding glucose and fructose?
Signup and view all the answers
Maltose, a dimer, is formed from two glucose molecules through which of these processes?
Maltose, a dimer, is formed from two glucose molecules through which of these processes?
Signup and view all the answers
What type of linkage is formed between sugar molecules during the synthesis of complex carbohydrates?
What type of linkage is formed between sugar molecules during the synthesis of complex carbohydrates?
Signup and view all the answers
Why can humans digest starch but not cellulose?
Why can humans digest starch but not cellulose?
Signup and view all the answers
What distinguishes monomers from polymers in the context of carbohydrates?
What distinguishes monomers from polymers in the context of carbohydrates?
Signup and view all the answers
Which type of fatty acids are primarily found in lipids that are solid at room temperature?
Which type of fatty acids are primarily found in lipids that are solid at room temperature?
Signup and view all the answers
What is the role of R-groups in amino acids?
What is the role of R-groups in amino acids?
Signup and view all the answers
What is the process called that creates ester bonds between fatty acids and glycerol in triglycerides?
What is the process called that creates ester bonds between fatty acids and glycerol in triglycerides?
Signup and view all the answers
Which type of fatty acids are typically found in liquid vegetable oils?
Which type of fatty acids are typically found in liquid vegetable oils?
Signup and view all the answers
What scientific term is used for many monomers linked together?
What scientific term is used for many monomers linked together?
Signup and view all the answers
What characterizes trans fats in relation to their natural occurrence?
What characterizes trans fats in relation to their natural occurrence?
Signup and view all the answers
Which configuration of fatty acids allows for a straight chain similar to saturated fatty acids?
Which configuration of fatty acids allows for a straight chain similar to saturated fatty acids?
Signup and view all the answers
Which group is identified as part of amino acids and is crucial for protein formation?
Which group is identified as part of amino acids and is crucial for protein formation?
Signup and view all the answers
What determines the function of a protein?
What determines the function of a protein?
Signup and view all the answers
Which type of bond holds the two strands of DNA together?
Which type of bond holds the two strands of DNA together?
Signup and view all the answers
What is the role of hemoglobin in the human body?
What is the role of hemoglobin in the human body?
Signup and view all the answers
What happens to a protein when it is denatured?
What happens to a protein when it is denatured?
Signup and view all the answers
Which of the following is NOT a function of proteins?
Which of the following is NOT a function of proteins?
Signup and view all the answers
What is the basic building block of nucleic acids?
What is the basic building block of nucleic acids?
Signup and view all the answers
Which protein is known to assist in muscle contraction?
Which protein is known to assist in muscle contraction?
Signup and view all the answers
What occurs when there is a mutation in the DNA code instructing protein synthesis?
What occurs when there is a mutation in the DNA code instructing protein synthesis?
Signup and view all the answers
Study Notes
Building Blocks of Life
- Biological molecules are composed mainly of carbon, hydrogen, oxygen, and a few other elements. These elements are fundamental to life as we know it, forming the basis of all organic compounds. Carbon is unique in its ability to form four covalent bonds with other atoms, allowing for a diverse range of molecular structures to exist. Hydrogen and oxygen play critical roles in forming water and organic molecules, while elements like nitrogen, phosphorus, and sulfur are also essential for certain biological functions.
- Monomers, like glucose, bond to form polymers, which are larger chain molecules such as complex carbohydrates. Monomers serve as building blocks for the synthesis of larger macromolecules, enabling the complexity of biological systems. This process allows for the storage and transfer of energy, structure, and function within cells, highlighting the intricate relationships between different types of molecules.
Atoms to Molecules
- Functional groups define how molecules interact with others. These specific groups of atoms impart certain chemical properties to the larger molecules they are a part of, influencing solubility, reactivity, and the overall behavior of the molecule in various biological contexts. Common functional groups include hydroxyl, carboxyl, amino, and phosphate groups, each contributing unique characteristics.
- Monosaccharides like glucose are the simplest form of carbohydrates. They are characterized by their single sugar unit structure and serve as primary energy sources for cells. Monosaccharides can combine through glycosidic bonds to form disaccharides and polysaccharides, broadening their functional roles in biological systems. Their chemical formula, typically represented as (CH₂O)n, where n is the number of carbons, illustrates their foundational role in more complex carbohydrates.
Dehydration Synthesis and Hydrolysis
- Dehydration synthesis bonds two glucose monomers to create maltose (a dimer). This process involves the removal of a water molecule, enabling the two hydroxyl groups from different monosaccharides to interact and form a covalent bond. The formation of maltose exemplifies how simpler molecules can combine to create larger and more complex structures, which serve various functions including energy storage and transport.
- Hydrolysis breaks down dimers like maltose back into monomers by adding water. This reaction is catalyzed by enzymes in biological systems, allowing for the release of energy stored in the bonds of complex carbohydrates when they are needed by the organism. This breakdown process is crucial for nutrient availability and energy production in living organisms.
Carbohydrates
- Glycosidic linkages form when two sugars bond. This type of covalent bond is specialized for connecting carbohydrate monomers, and its formation can vary based on the orientation of the involved hydroxyl groups. These linkages contribute to the physical properties and digestibility of the resulting carbohydrates.
- Complex carbohydrates, such as starch and cellulose, are formed from many sugar monomers. Starch, primarily found in plants, serves as an energy storage polysaccharide that can be broken down into glucose by humans and animals. In contrast, cellulose, a structural component in plant cell walls, provides rigidity and support but is not digestible due to its different glycosidic linkages, which require specific enzymes to break down.
- Starch is digestible, while cellulose is not, due to differences in molecular structure. The unique bonding patterns in cellulose create strong fibrils that humans lack the enzymes to digest, whereas the branching structure of starch allows for easier breakdown by digestive enzymes.
- Cellulose is a key component of the cell wall in plants; examples include hemp, cotton, and linen. These materials are important not only for plant structure and integrity but also for human industry, where their fibers are harvested and utilized for various applications including textiles, paper, and biodegradable products.
Lipids
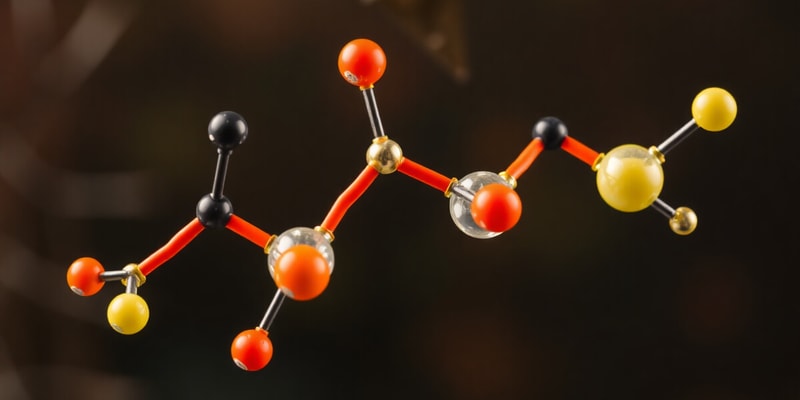
- Lipids include triglycerides, which are formed via ester bonds between fatty acids and glycerol. These molecules are crucial for energy storage, signaling, and cellular structure. In their triglyceride form, lipids can store more energy per gram than carbohydrates, making them an efficient energy source.
- Saturated fatty acids, like stearic acid, are
Studying That Suits You
Use AI to generate personalized quizzes and flashcards to suit your learning preferences.
Related Documents
Description
Test your knowledge on biological molecules, including their structures and functions. This quiz covers topics such as functional groups, monomers, and the processes of dehydration synthesis and hydrolysis. Ideal for students studying biology and the chemistry of life.