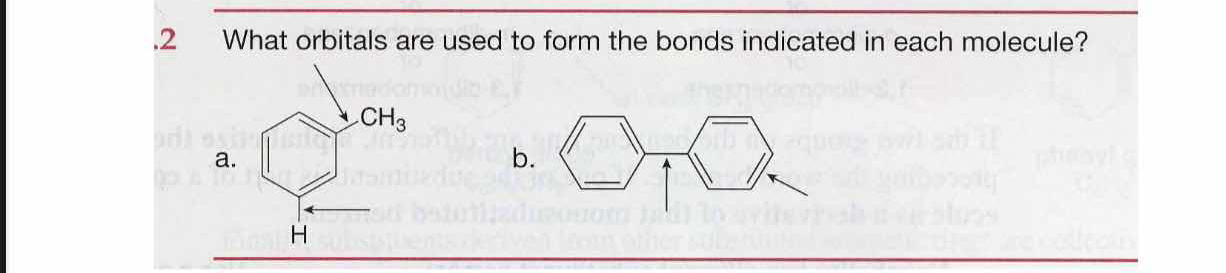
What orbitals are used to form the bonds indicated in each molecule?
Understand the Problem
The question is asking about the specific atomic orbitals involved in forming the bonds indicated in the provided molecular structures.
Answer
Bond a: C sp2-H 1s, C sp2-C sp3. Bond b: C sp2-C sp2.
Bond a: C-H bond uses C sp2 with H 1s, C-CH3 bond uses C sp2 with C sp3. Bond b: C-C bond uses C sp2 with C sp2.
Answer for screen readers
Bond a: C-H bond uses C sp2 with H 1s, C-CH3 bond uses C sp2 with C sp3. Bond b: C-C bond uses C sp2 with C sp2.
More Information
In benzene, carbon is sp2 hybridized, forming σ bonds with sp2-sp2 or sp2-1s orbitals. The methyl group has sp3 hybridization for the C-H bonds.
Tips
A common mistake is confusing the hybridization state of carbons in aromatic systems; remember that in benzene, carbons are sp2 hybridized.
Sources
- Hybrid Orbitals - Chemistry LibreTexts - chem.libretexts.org
- Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3 - YouTube - youtube.com
AI-generated content may contain errors. Please verify critical information