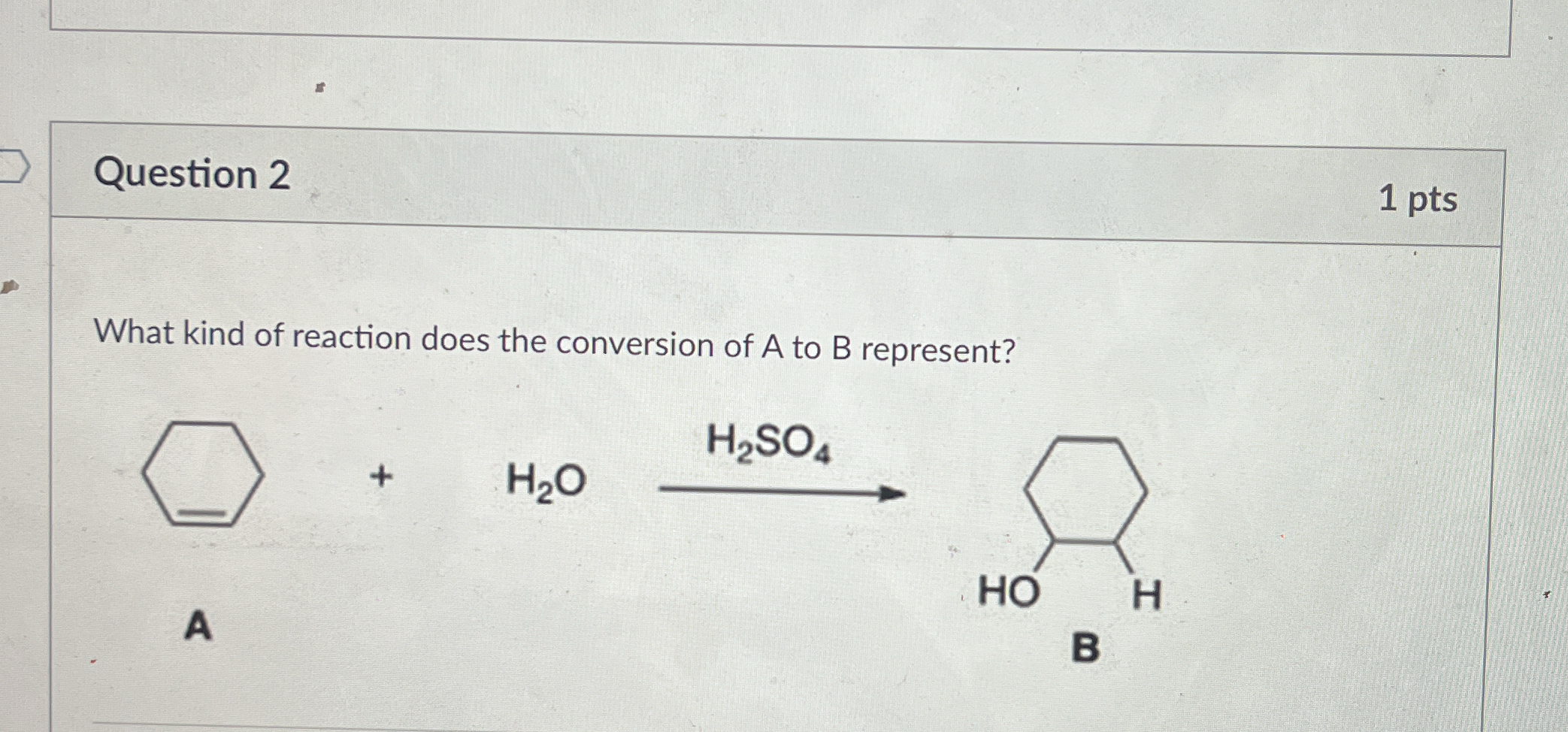
What kind of reaction does the conversion of A to B represent?
Understand the Problem
The question is asking what type of chemical reaction is represented by the conversion of compound A to compound B in the presence of sulfuric acid (H2SO4) and water (H2O). This involves determining the nature of the reaction based on the given reactants and products.
Answer
Addition reaction (hydration reaction).
The reaction represents an addition reaction, specifically an acid-catalyzed hydration of an alkene.
Answer for screen readers
The reaction represents an addition reaction, specifically an acid-catalyzed hydration of an alkene.
More Information
The conversion involves adding water (H2O) to an alkene in the presence of an acid, a common method for converting alkenes to alcohols.
Tips
A common mistake is confusing addition reactions with substitution reactions. Focus on the reagents and the type of bonds formed or broken.
Sources
- Addition reaction - Study.com - homework.study.com
AI-generated content may contain errors. Please verify critical information