What is the principle of the limit test of chloride?
Understand the Problem
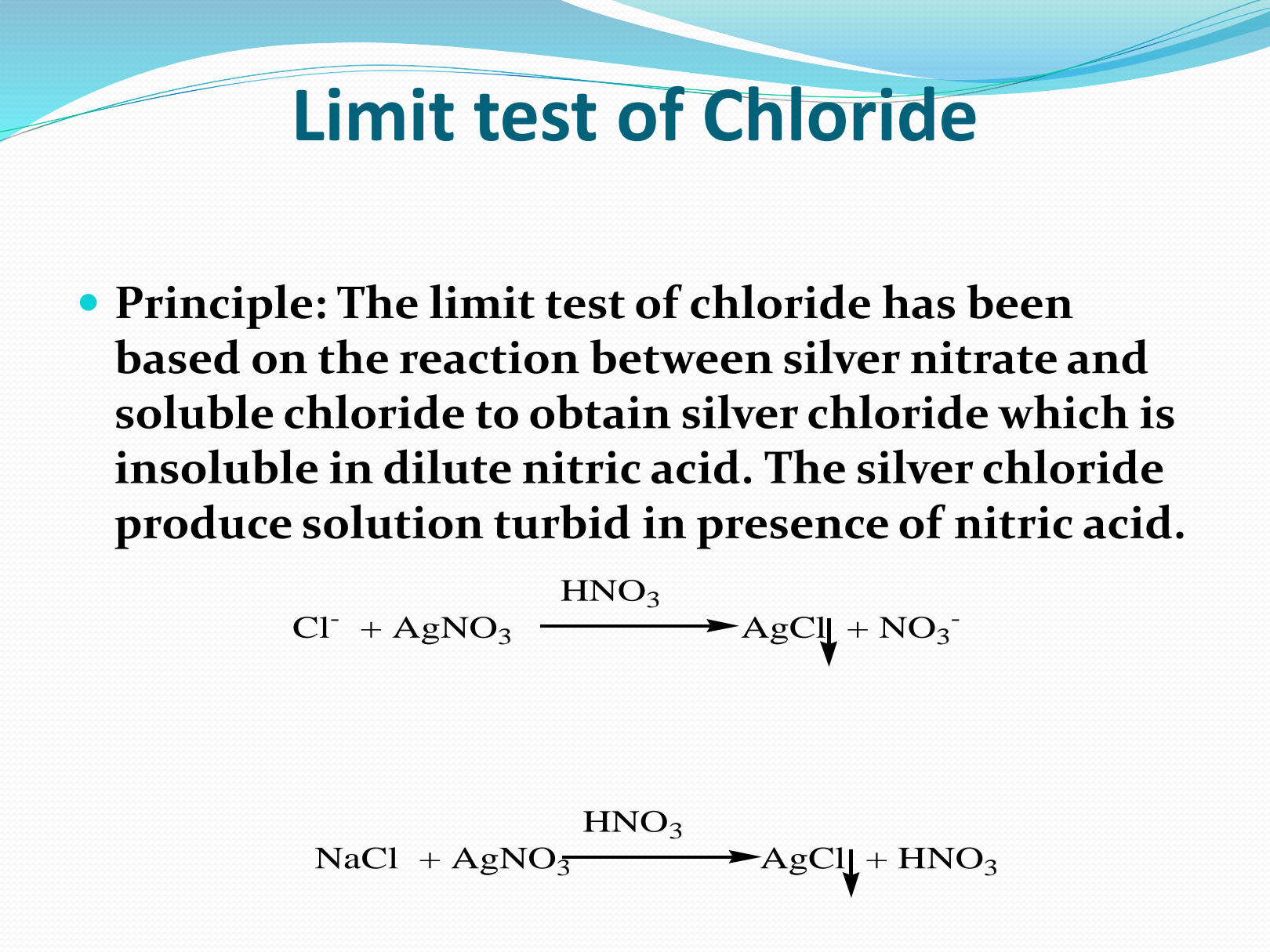
The question is about the principle behind the limit test for chloride, which involves a chemical reaction between silver nitrate and soluble chloride. This explains how silver chloride is formed and its properties in the presence of dilute nitric acid.
Answer
Reaction with silver nitrate forms insoluble silver chloride, causing turbidity.
The principle of the limit test for chloride is based on the reaction of soluble chloride with silver nitrate in dilute nitric acid to form silver chloride. This results in the formation of a turbid solution due to insoluble silver chloride particles.
Answer for screen readers
The principle of the limit test for chloride is based on the reaction of soluble chloride with silver nitrate in dilute nitric acid to form silver chloride. This results in the formation of a turbid solution due to insoluble silver chloride particles.
More Information
The test relies on the formation of a white precipitate of silver chloride. The extent of turbidity indicates the amount of chloride.
Tips
Ensure to add dilute nitric acid to prevent the precipitation of other metals that might cause interference.
Sources
- Principle of the Limit Test of Chloride - asianjpr.com
- What is the Principle of Limit Test for Chloride? - BYJU'S - byjus.com
- Limit Test of Chloride - Web Formulas - web-formulas.com
AI-generated content may contain errors. Please verify critical information