What are the expected products of the reaction when a compound with a hydroxyl group and an amino group is treated with HNO2 followed by H3O+?
Understand the Problem
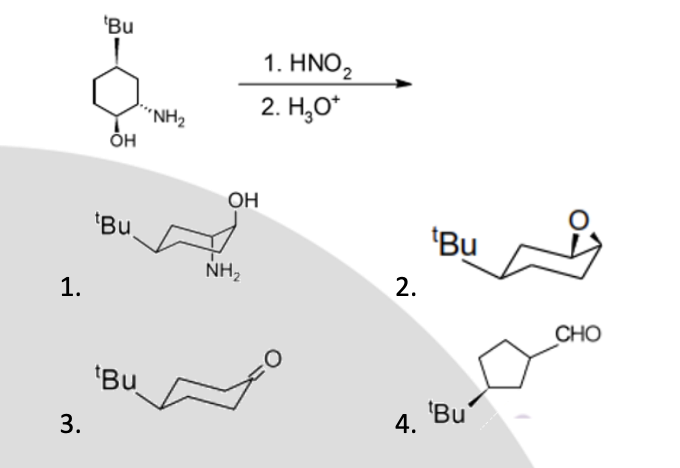
The question is asking about a chemical reaction involving a compound with a hydroxyl group (OH) and an amino group (NH2) being treated with nitrous acid (HNO2) followed by hydronium ion (H3O+), likely to determine the expected products from this reaction sequence.
Answer
Structure 2 is the expected product.
The expected product is a ketone, which is structure 2.
Answer for screen readers
The expected product is a ketone, which is structure 2.
More Information
In this reaction, the amino group is converted first into a diazonium salt and subsequently replaced by a hydroxyl group, resulting in a ketone.
Tips
A common mistake is overlooking the conversion of the diazonium group to a hydroxyl group under acidic conditions.
Sources
- Reaction of Amines with Nitrous Acid - Chemistry LibreTexts - chem.libretexts.org
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information