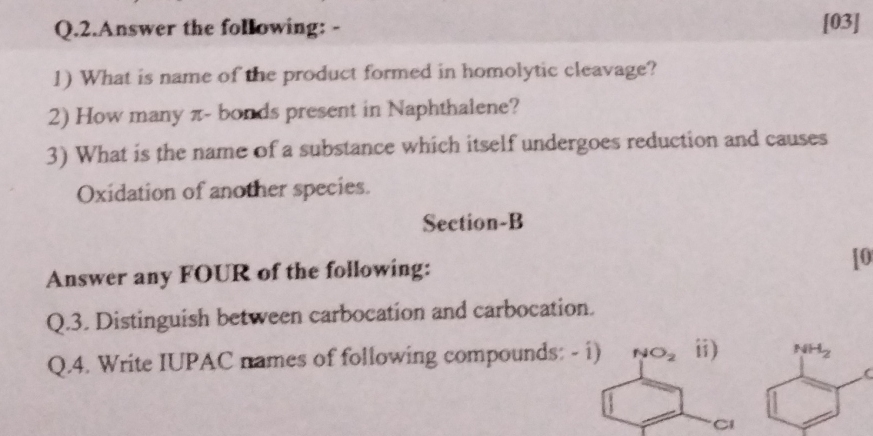
1) What is the name of the product formed in homolytic cleavage? 2) How many π-bonds are present in Naphthalene? 3) What is the name of a substance which itself undergoes reduction... 1) What is the name of the product formed in homolytic cleavage? 2) How many π-bonds are present in Naphthalene? 3) What is the name of a substance which itself undergoes reduction and causes oxidation of another species? 4) Distinguish between carbocation and carbanion. 5) Write IUPAC names of the following compounds: i) 2-nitrochlorobenzene ii) 4-aminobenzenesulfonic acid.
Understand the Problem
The question is asking for specific information related to organic chemistry concepts, particularly about products of reactions, molecular structure, and IUPAC nomenclature. It also includes a request to distinguish between two types of organic species (carbocation) and requires naming certain compounds.
Answer
1) Radical 2) 5 π-bonds 3) Oxidizing agent 4) Carbocations are positive, carbanions negative 5) i) 1-chloro-2-nitrobenzene ii) 4-aminobenzenesulfonic acid
- The product formed in homolytic cleavage is a radical. 2) Naphthalene has 5 π-bonds. 3) The substance is called an oxidizing agent. 4) Carbocations are positively charged carbon ions; carbanions are negatively charged carbon ions. 5) i) 1-chloro-2-nitrobenzene; ii) 4-aminobenzenesulfonic acid.
Answer for screen readers
- The product formed in homolytic cleavage is a radical. 2) Naphthalene has 5 π-bonds. 3) The substance is called an oxidizing agent. 4) Carbocations are positively charged carbon ions; carbanions are negatively charged carbon ions. 5) i) 1-chloro-2-nitrobenzene; ii) 4-aminobenzenesulfonic acid.
More Information
Radicals are highly reactive due to unpaired electrons, and naphthalene's aromatic structure involves a combination of π-bonds that contribute to its stability.
Tips
Common mistakes include miscounting π-bonds or confusing oxidizing and reducing agents.
Sources
- Homolytic & Heterolytic Fission of Covalent Bonds - BYJU'S - byjus.com
- Rules for Aromaticity: The 4 Key Factors - Master Organic Chemistry - masterorganicchemistry.com
AI-generated content may contain errors. Please verify critical information