What are the classifications of elements in terms of metals and nonmetals, and what are their properties?
Understand the Problem
The question seems to be related to the classification of elements, specifically focusing on metals and nonmetals, and their characteristics. It aims to assess understanding of basic chemistry concepts related to the properties of different elements.
Answer
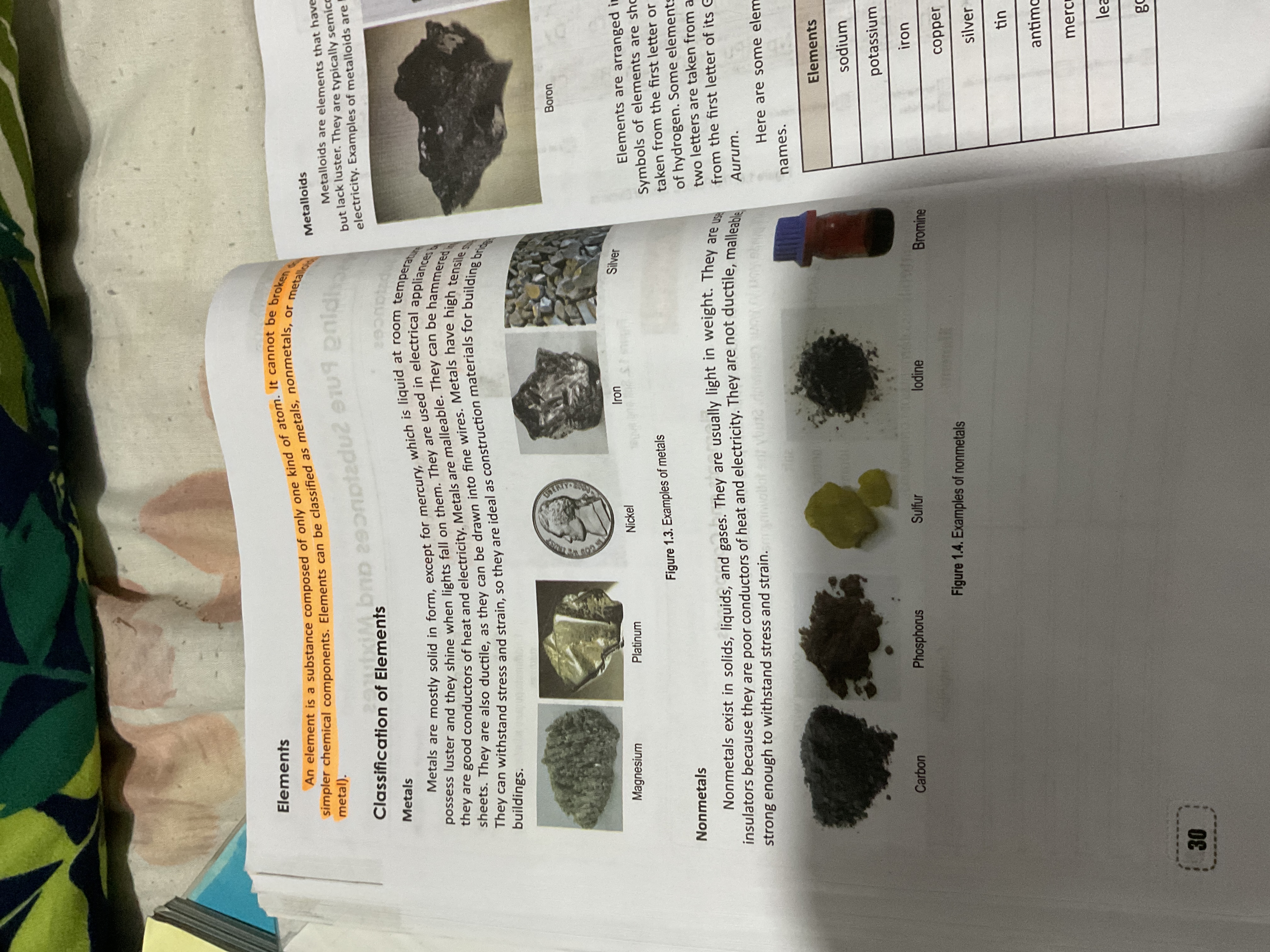
Elements are classified as metals, nonmetals, and metalloids. Metals: solid, malleable, ductile, conductive, lustrous. Nonmetals: various states, poor conductors, brittle, non-lustrous. Metalloids: intermediate properties.
The classifications of elements are metals, nonmetals, and metalloids. Metals are solid (except mercury), malleable, ductile, conduct heat and electricity, and exhibit luster. Nonmetals exist in various states, are poor conductors, brittle, and do not exhibit luster. Metalloids have properties intermediate between metals and nonmetals.
Answer for screen readers
The classifications of elements are metals, nonmetals, and metalloids. Metals are solid (except mercury), malleable, ductile, conduct heat and electricity, and exhibit luster. Nonmetals exist in various states, are poor conductors, brittle, and do not exhibit luster. Metalloids have properties intermediate between metals and nonmetals.
More Information
Metals have high tensile strength and are used in construction due to their ability to withstand stress and strain. Nonmetals, being poor conductors, are often used as insulators. Metalloids have varied applications due to their intermediate properties, such as in semiconductors.
Tips
A common mistake is to assume all elements fit neatly into these categories; some elements may have mixed properties of metals and nonmetals.
Sources
- 2.11: Metals, Nonmetals, and Metalloids - chem.libretexts.org
- Classification of Elements: Metals, Non Metals, and Metalloids - Turito - turito.com
- The Periodic Table: Metals, Nonmetals, and Metalloids - dummies - dummies.com
AI-generated content may contain errors. Please verify critical information