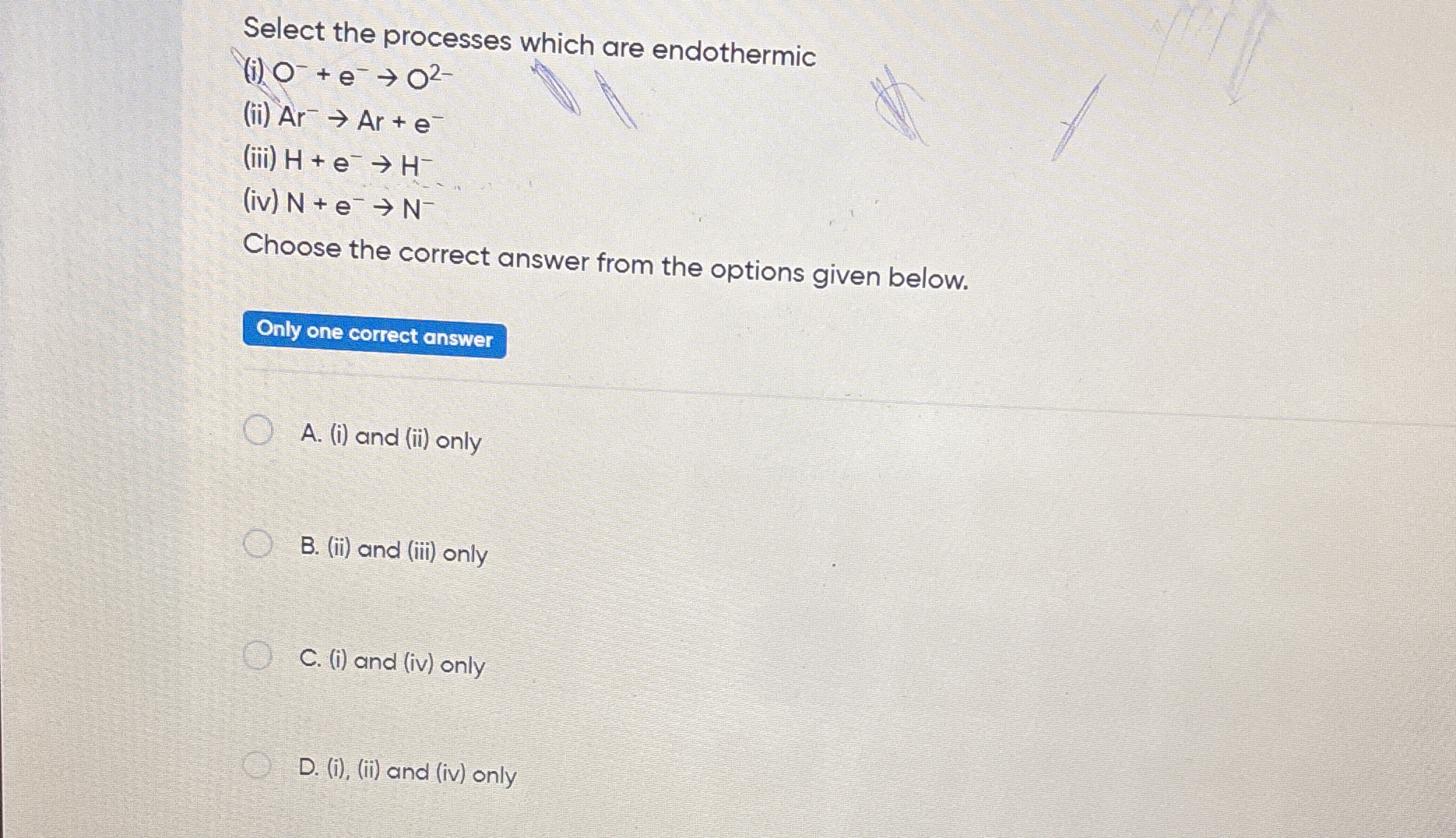
Select the processes which are endothermic (i) O + e -> O2- (ii) Ar -> Ar + e- (iii) H + e -> H- (iv) N + e -> N- Choose the correct answer from the options given below.
Understand the Problem
The question is asking to identify which listed processes are endothermic. It presents four processes and asks to select the correct one from given options.
Answer
A. (i) and (ii) only
The processes (i) O + e⁻ → O²⁻ and (ii) Ar → Ar⁺ + e⁻ are endothermic.
Answer for screen readers
The processes (i) O + e⁻ → O²⁻ and (ii) Ar → Ar⁺ + e⁻ are endothermic.
More Information
Endothermic processes require an input of energy to proceed, like ionization or the addition of a second electron to an already negative ion.
Tips
A common mistake is confusing electron affinity with ionization energy. Electron affinity releases energy when adding an electron, while ionization energy absorbs energy to remove an electron.
Sources
- Endothermic vs. exothermic reactions (article) - Khan Academy - khanacademy.org
- Which of the following process involves absorption of energy? - toppr.com
AI-generated content may contain errors. Please verify critical information