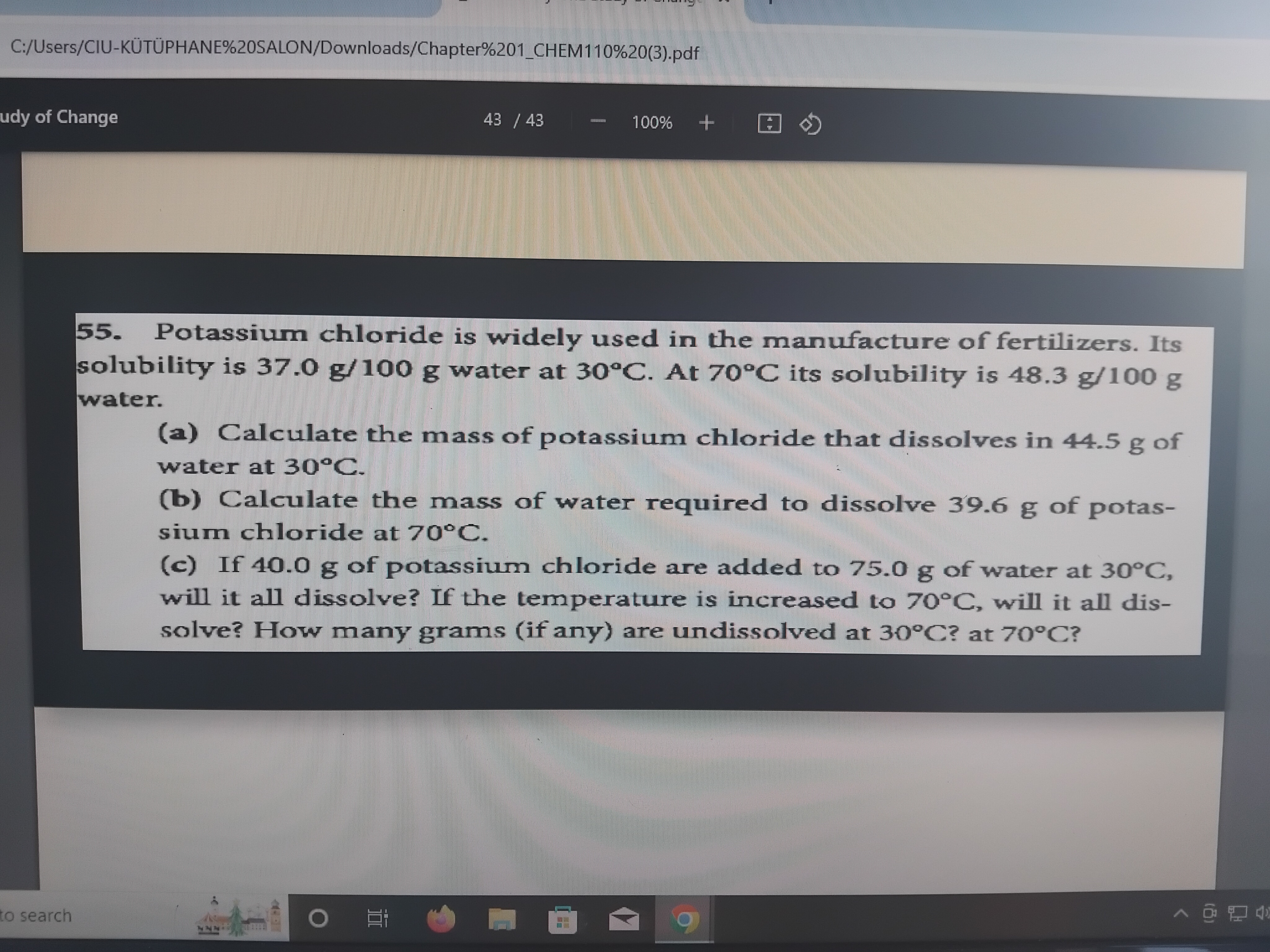
Potassium chloride is widely used in the manufacture of fertilizers. Its solubility is 37.0 g/100 g water at 30°C. At 70°C its solubility is 48.3 g/100 g water. (a) Calculate the m... Potassium chloride is widely used in the manufacture of fertilizers. Its solubility is 37.0 g/100 g water at 30°C. At 70°C its solubility is 48.3 g/100 g water. (a) Calculate the mass of potassium chloride that dissolves in 44.5 g of water at 30°C. (b) Calculate the mass of water required to dissolve 39.6 g of potassium chloride at 70°C. (c) If 40.0 g of potassium chloride are added to 75.0 g of water at 30°C, will it all dissolve? If the temperature is increased to 70°C, will it all dissolve? How many grams (if any) are undissolved at 30°C? at 70°C?
Understand the Problem
The question is asking for calculations related to the solubility of potassium chloride in water at two different temperatures. It consists of three parts: calculating the mass of potassium chloride that dissolves in a certain amount of water at a specified temperature, determining the mass of water needed to dissolve a given amount of potassium chloride at another temperature, and analyzing the dissolution of potassium chloride when mixed with water and changing the temperature.
Answer
(a) 16.465 g, (b) 81.92 g, (c) 12.25 g undissolved at 30°C and 3.775 g undissolved at 70°C.
Answer for screen readers
- (a) 16.465 g of potassium chloride will dissolve in 44.5 g of water at 30°C.
- (b) 81.92 g of water is required to dissolve 39.6 g of potassium chloride at 70°C.
- (c) At 30°C, 12.25 g of KCl remains undissolved, and at 70°C, 3.775 g remains undissolved.
Steps to Solve
- Calculate mass of potassium chloride at 30°C
To find the mass of potassium chloride that dissolves in 44.5 g of water at 30°C, we use the solubility information. At 30°C, the solubility of potassium chloride is 37.0 g per 100 g of water.
Using the proportion: [ \text{Mass of KCl} = \left( \frac{37.0 , \text{g KCl}}{100 , \text{g water}} \right) \times 44.5 , \text{g water} ]
Calculating this gives: [ \text{Mass of KCl} = \frac{37.0 \times 44.5}{100} = 16.465 , \text{g} ]
- Calculate mass of water required at 70°C
Now, we need to find the mass of water required to dissolve 39.6 g of potassium chloride at 70°C. The solubility at this temperature is 48.3 g per 100 g of water.
Using the proportion: [ \text{Mass of water} = \left( \frac{100 , \text{g water}}{48.3 , \text{g KCl}} \right) \times 39.6 , \text{g KCl} ]
Calculating this gives: [ \text{Mass of water} = \frac{100 \times 39.6}{48.3} \approx 81.92 , \text{g} ]
- Determine if all KCl dissolves at different temperatures
For part (c), we need to check if 40.0 g of KCl will dissolve in 75.0 g of water at 30°C. From earlier, we know that:
- The maximum amount of KCl that can dissolve in 75.0 g of water is: [ \text{Maximum KCl} = \left( \frac{37.0 , \text{g KCl}}{100 , \text{g water}} \right) \times 75.0 , \text{g water = 27.75 , g KCl} ]
Since 40.0 g > 27.75 g, it will not dissolve completely at 30°C.
Next, we check if the remaining KCl dissolves at 70°C. The maximum KCl that can dissolve in 75.0 g of water at 70°C is: [ \text{Maximum KCl at 70°C} = \left( \frac{48.3 , \text{g KCl}}{100 , \text{g water}} \right) \times 75.0 , \text{g water \approx 36.225 , g KCl} ]
Since 40.0 g > 36.225 g, some KCl will remain undissolved at 70°C as well.
- Determine undissolved KCl at both temperatures
Total KCl added = 40.0 g
At 30°C:
- Dissolved KCl = 27.75 g
- Undissolved KCl = (40.0 , \text{g} - 27.75 , \text{g} = 12.25 , \text{g})
At 70°C:
- Dissolved KCl = 36.225 g
- Undissolved KCl = (40.0 , \text{g} - 36.225 , \text{g} = 3.775 , \text{g})
- (a) 16.465 g of potassium chloride will dissolve in 44.5 g of water at 30°C.
- (b) 81.92 g of water is required to dissolve 39.6 g of potassium chloride at 70°C.
- (c) At 30°C, 12.25 g of KCl remains undissolved, and at 70°C, 3.775 g remains undissolved.
More Information
This exercise illustrates the concepts of solubility at varying temperatures, an important principle in chemistry that affects how substances behave in solution. Potassium chloride serves as a common example due to its practical applications.
Tips
- Miscalculating the solubility values or proportions when finding the amounts that can dissolve. Always ensure to use proper ratios based on the information given.
AI-generated content may contain errors. Please verify critical information