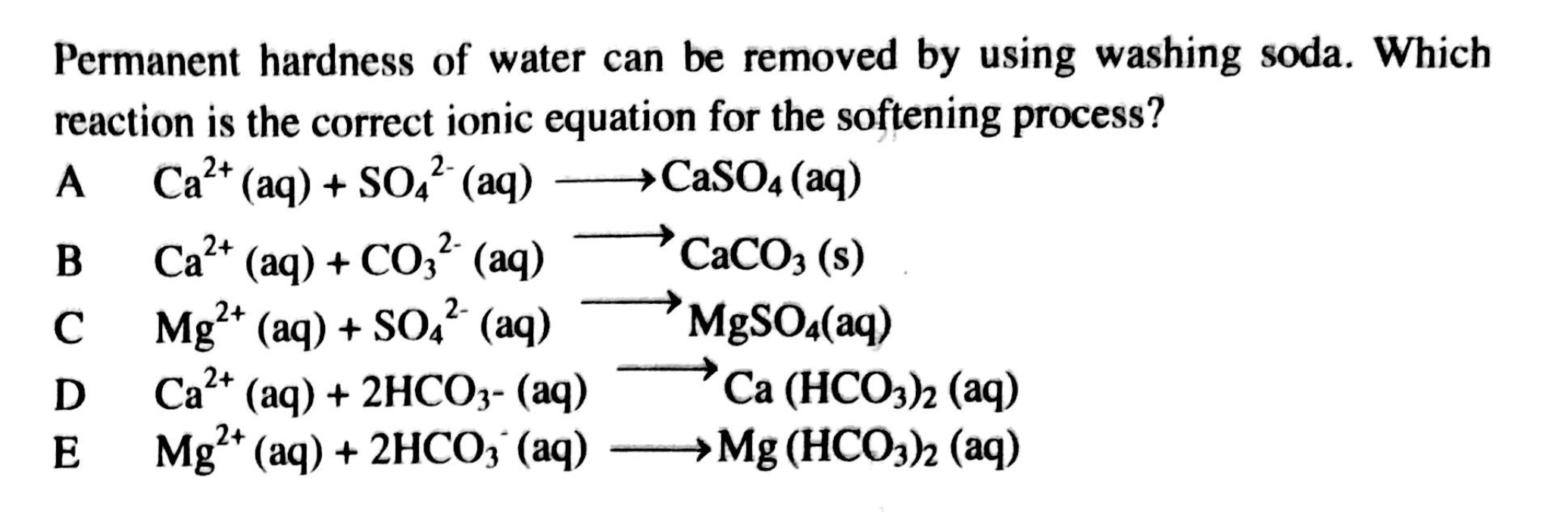
Permanent hardness of water can be removed by using washing soda. Which reaction is the correct ionic equation for the softening process?
Understand the Problem
The question is asking for the correct ionic equation involved in the process of softening water using washing soda. It provides several options that represent different chemical reactions, and we need to identify which one is accurate.
Answer
B: Ca²⁺ (aq) + CO₃²⁻ (aq) → CaCO₃ (s)
The final answer is B: Ca²⁺ (aq) + CO₃²⁻ (aq) → CaCO₃ (s)
Answer for screen readers
The final answer is B: Ca²⁺ (aq) + CO₃²⁻ (aq) → CaCO₃ (s)
More Information
Permanent hardness caused by calcium and magnesium ions is removed by forming insoluble carbonates using washing soda, Na₂CO₃.
Tips
A common mistake is choosing reactions that don't form insoluble products. Focus on the formation of an insoluble carbonate.
Sources
AI-generated content may contain errors. Please verify critical information