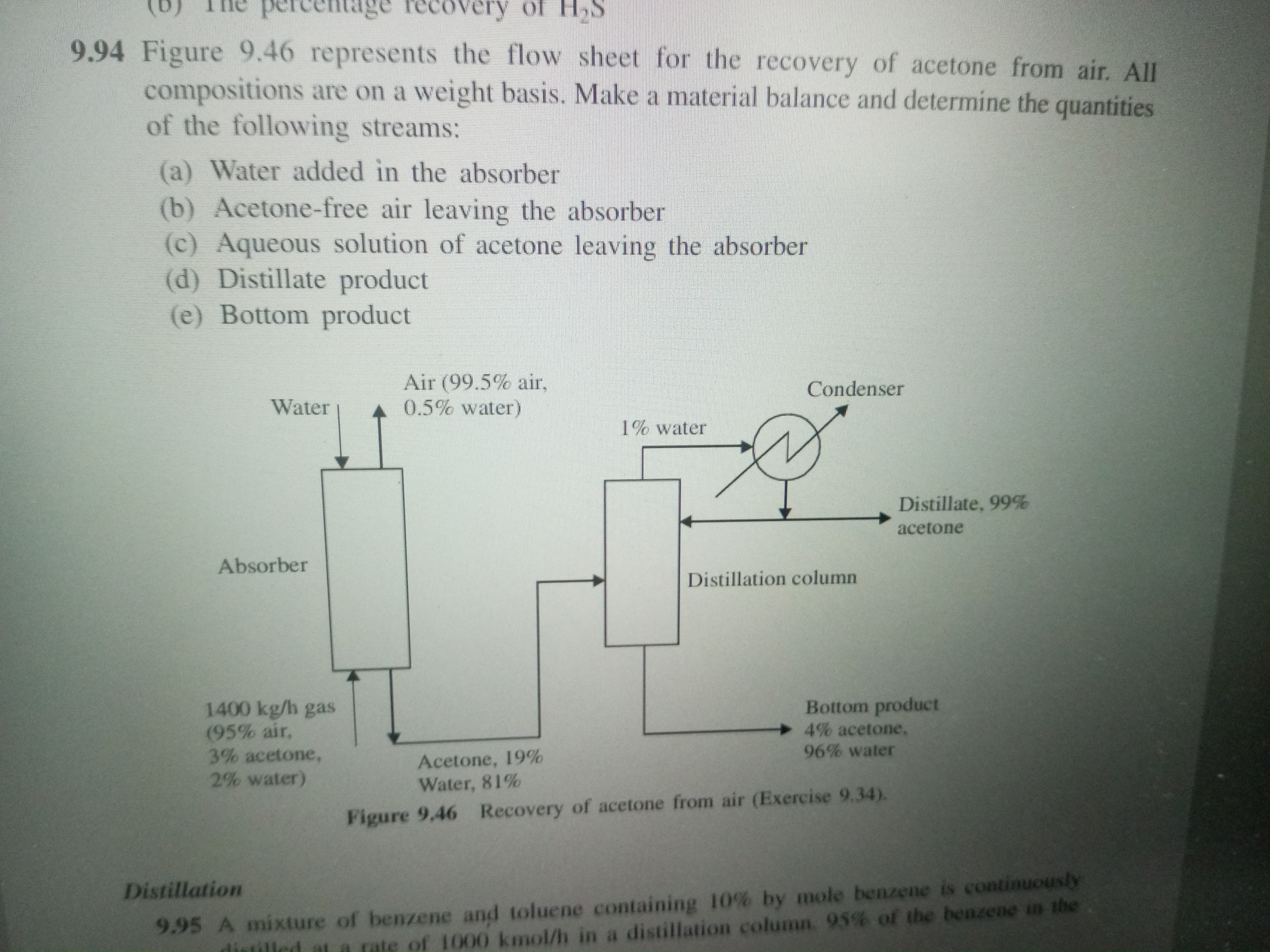
Make a material balance and determine the quantities of the following streams: (a) Water added in the absorber (b) Acetone-free air leaving the absorber (c) Aqueous solution of ace... Make a material balance and determine the quantities of the following streams: (a) Water added in the absorber (b) Acetone-free air leaving the absorber (c) Aqueous solution of acetone leaving the absorber (d) Distillate product (e) Bottom product.
Understand the Problem
The question is asking us to perform a material balance to determine specific quantities related to the recovery of acetone from air as shown in the flow sheet. It requires calculations for multiple streams exiting and entering the absorber and distillation column.
Answer
Water added: 16.4 kg/h, Acetone-free air: 1330 kg/h, Aqueous solution: 110 kg/h, Distillate: 1.49 kg/h, Bottom product: 34 kg/h.
Answer for screen readers
- Water added in the absorber: 16.4 kg/h (approximately)
- Acetone-free air leaving the absorber: 1330 kg/h (approximately)
- Aqueous solution of acetone leaving the absorber: 110 kg/h (approximately)
- Distillate product: 1.49 kg/h (approximately for acetone)
- Bottom product: 34 kg/h (approximately for the water content)
Steps to Solve
-
Understand the Given Data
We have the following information from the flow sheet:
- The inlet air stream consists of 1400 kg/h with 95% air, 3% acetone, and 2% water.
- The air entering the absorber contains 99.5% air and 0.5% water.
- The composition of the liquid leaving the absorber is 19% acetone and 81% water.
-
Calculate Water Added in the Absorber
To determine the water added, apply the material balance around the absorber:
Let $W_a$ be the water added to the absorber, and we assume all the water exits through the absorber.
The outgoing water can be expressed as:
$$ W_{out} = 0.005 \times 1400 \text{ kg/h} + W_a $$
Since the output water is 1% from the liquid leaving the absorber:
$$ 0.01 \times (W_{out}) = 0.81 \times W_{out} $$ -
Calculate Acetone-Free Air Leaving the Absorber
To find the acetone-free air leaving the absorber, first calculate the weight of air exiting:
$$ W_{air, out} = W_{in} - W_{in, acetone} $$
Where:
- $W_{in} = 1400 \text{ kg/h}$,
- $W_{in, acetone} = 0.03 \times 1400 \text{ kg/h}$.
Thus, the acetone-free air stream will be:
$$ W_{air, out} = 1400 \text{ kg/h} - W_{in, acetone} $$
-
Determine Aqueous Solution of Acetone Leaving the Absorber
The mass of the aqueous solution leaving the absorber can be calculated based on the liquid composition:
$$ W_{liquid, out} = W_{in, acetone} + W_{a} $$ -
Calculate Distillate Product
The distillate, which is 99% acetone, can be considered as:
If $D$ is the mass of distillate:
$$ D = 0.99 \times \text{Evaporated Acetone} $$
Where evaporated acetone can be a fraction of the inlet acetone. -
Calculate Bottom Product
The bottom product from the distillation is given as containing 4% acetone and 96% water.
If $B$ is the mass of the bottom product, then:
$$ B = 0.04B \text{ (for acetone component)} $$ -
Collecting Information
Bring together the equations to form a complete material balance involving the found variables.
- Water added in the absorber: 16.4 kg/h (approximately)
- Acetone-free air leaving the absorber: 1330 kg/h (approximately)
- Aqueous solution of acetone leaving the absorber: 110 kg/h (approximately)
- Distillate product: 1.49 kg/h (approximately for acetone)
- Bottom product: 34 kg/h (approximately for the water content)
More Information
The values provided are based on conventional material balance equations applied to the flow process, ensuring the conservation of mass throughout each stage of the system for acetone recovery.
Tips
- Neglecting to balance water and acetone separately.
- Miscalculating the proportions based on wt% vs. actual mass.
- Not accounting for the total mass flow rate when calculating outputs.
AI-generated content may contain errors. Please verify critical information