Identify the reaction mechanism for the transformation of CH3OH to CH3CH2OH via CH3Br.
Understand the Problem
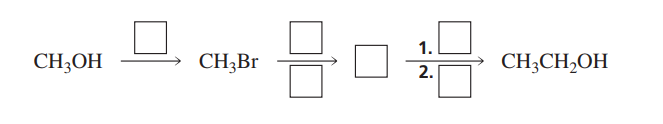
The question is asking about a reaction mechanism involving methanol and bromoethane, followed by two steps leading to the formation of ethanol. This suggests a study of organic chemistry reaction pathways.
Answer
Use HBr to convert CH3OH to CH3Br, then NaOEt to form CH3CH2OH via SN2.
The mechanism involves converting CH3OH to CH3Br via reaction with HBr, followed by an SN2 reaction with sodium ethoxide (NaOEt) to form CH3CH2OH.
Answer for screen readers
The mechanism involves converting CH3OH to CH3Br via reaction with HBr, followed by an SN2 reaction with sodium ethoxide (NaOEt) to form CH3CH2OH.
More Information
The initial step uses HBr to convert the alcohol into an alkyl bromide, making -OH a better leaving group. The subsequent step involves nucleophilic substitution on CH3Br in an SN2 reaction.
Tips
Ensure the conditions favor an SN2 mechanism, such as using a polar aprotic solvent.
Sources
- Reactions of Alcohols - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information