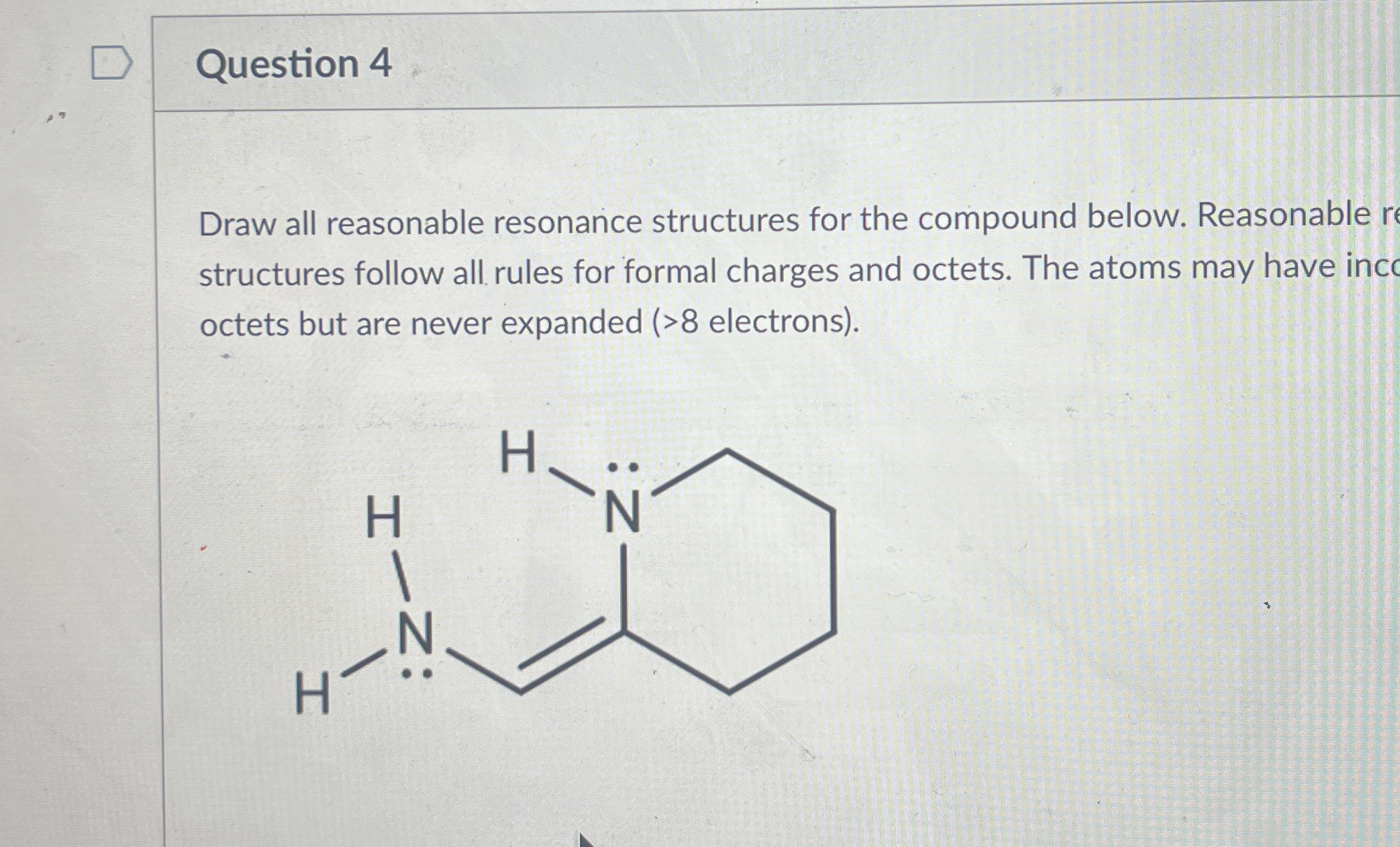
Draw all reasonable resonance structures for the compound below. Reasonable resonance structures follow all rules for formal charges and octets. The atoms may have incomplete octet... Draw all reasonable resonance structures for the compound below. Reasonable resonance structures follow all rules for formal charges and octets. The atoms may have incomplete octets but are never expanded (>8 electrons).
Understand the Problem
The question is asking for the drawing of all reasonable resonance structures for a given chemical compound, adhering to the rules of formal charges and octets without expanding any octets beyond 8 electrons.
Answer
Resonance structures include: 1. A structure with one nitrogen double-bonded to carbon and having a lone pair. 2. A structure with the second nitrogen double-bonded instead, ensuring no atom exceeds 8 electrons.
Answer for screen readers
The reasonable resonance structures for the compound consist of:
- Structure where a nitrogen atom has a double bond with the adjacent carbon, and the other nitrogen holds a lone pair.
- Structure where the positions of double bonds swap between nitrogen atoms without exceeding octet.
Steps to Solve
-
Identify the Atoms and Bonds Begin by identifying all the atoms in the compound: there are hydrogen (H) and nitrogen (N) atoms, along with a carbon (C) ring.
-
Count Valence Electrons Calculate the total number of valence electrons:
- Hydrogen has 1 electron each, contributing 4 from 4 H atoms ($4 \times 1 = 4$).
- Nitrogen has 5 electrons each, contributing 10 from 2 N atoms ($2 \times 5 = 10$).
- Each carbon in the ring has 4 electrons, contributing 20 from 5 carbon atoms ($5 \times 4 = 20$).
Total valence electrons: $$4 + 10 + 20 = 34$$
-
Draw the Initial Structure Construct the basic structure using the identified atoms and count the electrons utilized.
-
Distribute Electrons to Follow Octet Rule Adjust the electrons to ensure that each atom follows the octet rule where applicable. The nitrogen atom will have a lone pair contributing to resonance.
-
Create Resonance Structures Generate resonance structures by moving the lone pair electrons on nitrogen to form double bonds with adjacent atoms and checking for alternative arrangements without exceeding the octet.
-
Check Formal Charges Calculate formal charges for each resonance structure to ensure all atoms are stable: $$ \text{Formal Charge} = \text{Valence Electrons} - \left(\text{Non-bonding Electrons} + \frac{1}{2} \times \text{Bonding Electrons}\right) $$
-
Final Verification Verify that no atom has more than 8 electrons in the final structures, then list all valid resonance structures.
The reasonable resonance structures for the compound consist of:
- Structure where a nitrogen atom has a double bond with the adjacent carbon, and the other nitrogen holds a lone pair.
- Structure where the positions of double bonds swap between nitrogen atoms without exceeding octet.
More Information
Resonance structures illustrate different bonding arrangements in molecules, reflecting how electrons are distributed differently around the atoms, hence stabilizing molecules.
Tips
- Forgetting to check for formal charges which can lead to selecting an unrealistic resonance structure.
- Moving electrons without maintaining the octet rule for nitrogen or carbon.
AI-generated content may contain errors. Please verify critical information