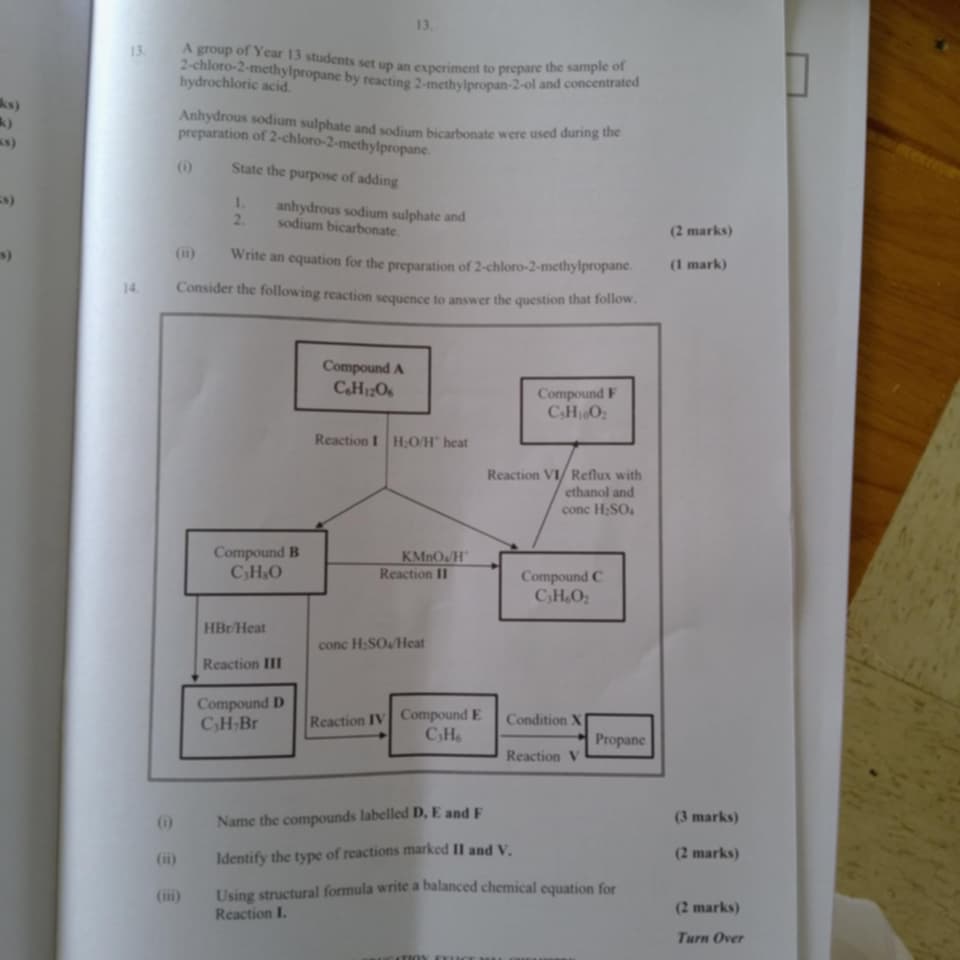
A group of Year 13 students set up an experiment to prepare the sample of 2-chloro-2-methylpropane by reacting 2-methylpropan-2-ol and concentrated hydrochloric acid. Anhydrous sod... A group of Year 13 students set up an experiment to prepare the sample of 2-chloro-2-methylpropane by reacting 2-methylpropan-2-ol and concentrated hydrochloric acid. Anhydrous sodium sulphate and sodium bicarbonate were used during the preparation. (i) State the purpose of adding 1. anhydrous sodium sulphate and 2. sodium bicarbonate. (2 marks) (ii) Write an equation for the preparation of 2-chloro-2-methylpropane. (1 mark) Consider the following reaction sequence to answer the question that follows. Name the compounds labelled D, E and F (3 marks), Identify the type of reactions marked II and V. (2 marks) Using structural formula write a balanced chemical equation for Reaction I. (2 marks)
Understand the Problem
The question involves understanding a chemistry experiment. It asks for the purpose of adding certain compounds, writing an equation for a specific preparation, identifying compounds in a reaction sequence, identifying types of reactions, and writing a balanced chemical equation. This requires knowledge of organic chemistry and reaction mechanisms.
Answer
1. Sodium sulphate dries the product. 2. Sodium bicarbonate neutralizes acid. Equation: (CH3)3COH + HCl → (CH3)3CCl + H2O.
(i) 1. Anhydrous sodium sulphate is used to dry the organic layer by removing water. 2. Sodium bicarbonate neutralizes any remaining acid. (ii) (CH3)3COH + HCl → (CH3)3CCl + H2O
Answer for screen readers
(i) 1. Anhydrous sodium sulphate is used to dry the organic layer by removing water. 2. Sodium bicarbonate neutralizes any remaining acid. (ii) (CH3)3COH + HCl → (CH3)3CCl + H2O
More Information
Anhydrous sodium sulphate is commonly used to dry organic solvents. Sodium bicarbonate is a mild base that reacts with residual HCl to prevent unwanted acidic conditions.
Tips
A common mistake is using too much sodium bicarbonate, leading to excess basic conditions. Ensure balanced stoichiometry when writing chemical equations.
Sources
- Core practical 6: Investigating chlorination of 2-methylpropan-2-ol - qualifications.pearson.com
- Experiment 5: Preparation of 2-chloro-2-methylpropane - chem.uiuc.edu