Understand the Problem
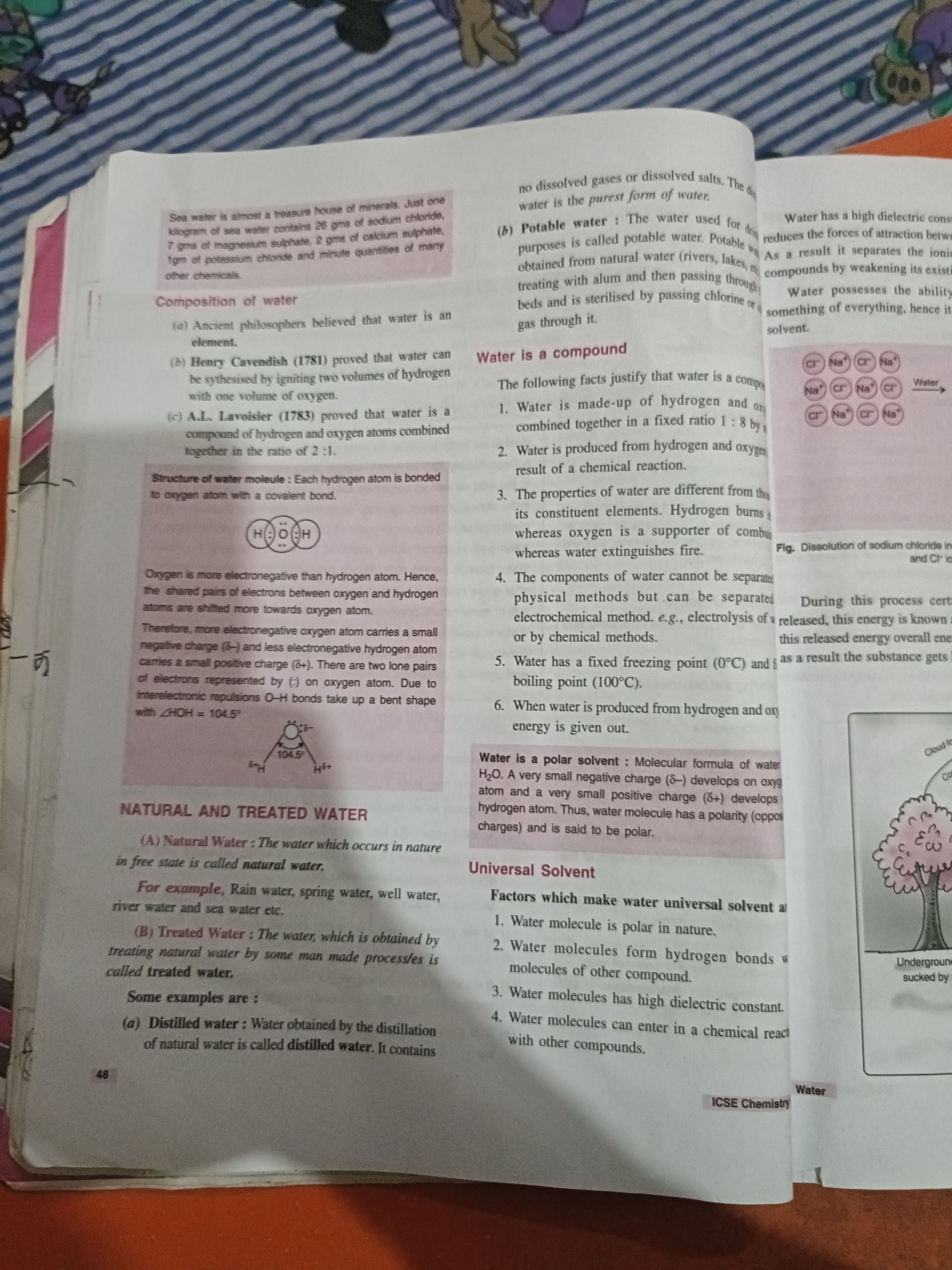
The text discusses the composition and properties of water, detailing its molecular structure, types (natural and treated), and its universal solvent capabilities.
Answer
Water is a universal solvent because it is polar, forms hydrogen bonds, has high dielectric constant, and can engage in chemical reactions.
Water is a universal solvent because it is polar in nature, can form hydrogen bonds with other compounds, has high dielectric constant, and can enter in chemical reactions.
Answer for screen readers
Water is a universal solvent because it is polar in nature, can form hydrogen bonds with other compounds, has high dielectric constant, and can enter in chemical reactions.
More Information
Water's polarity and hydrogen bonding capabilities make it excellent at dissolving a wide variety of substances, which is why it is often referred to as a 'universal solvent.'
AI-generated content may contain errors. Please verify critical information