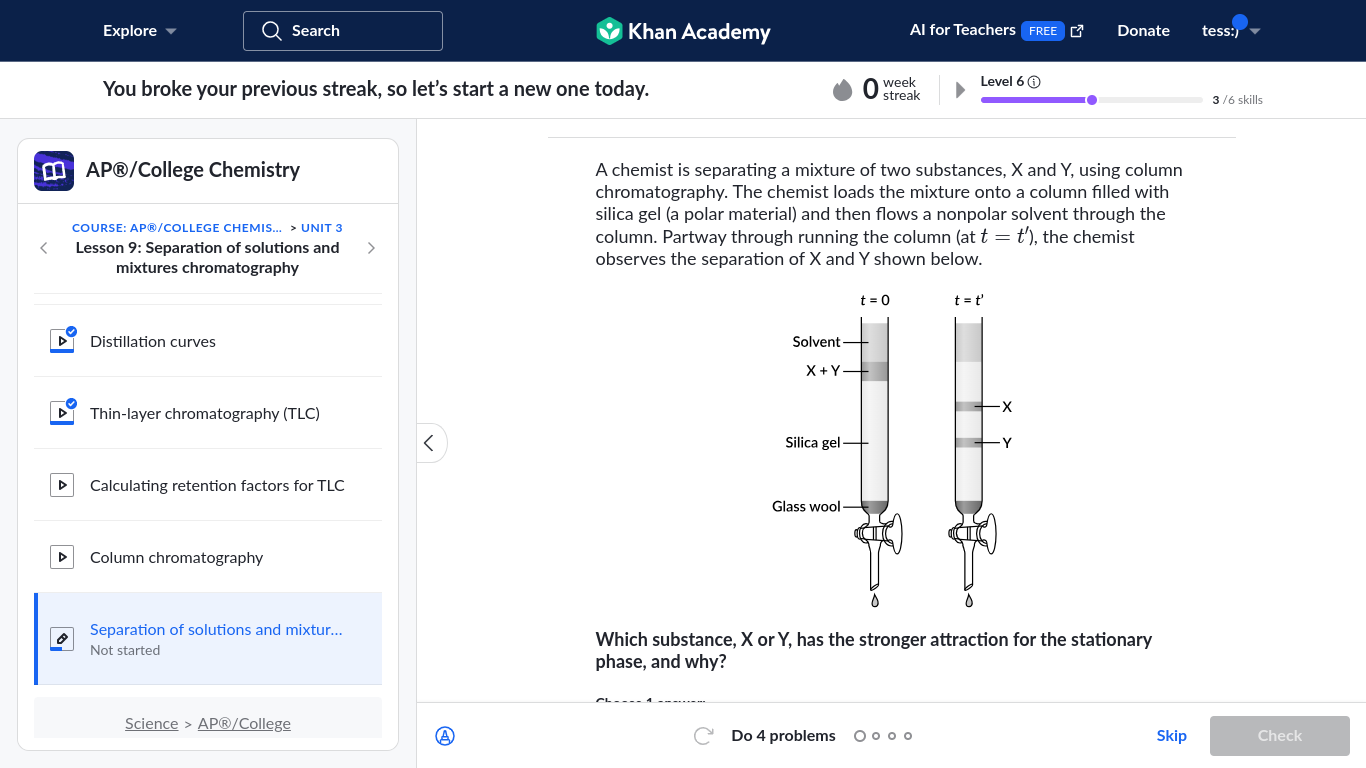
Which substance, X or Y, has the stronger attraction for the stationary phase, and why?
Understand the Problem
The question is asking which substance, X or Y, has a stronger attraction for the stationary phase in a chromatography setup. This involves understanding the principles of chromatography, where the interaction between substances and the stationary phase determines their separation.
Answer
Y has the stronger attraction because it remains closer to the polar stationary phase.
The final answer is Y, because it remains closer to the stationary phase, indicating a stronger attraction to the polar silica gel.
Answer for screen readers
The final answer is Y, because it remains closer to the stationary phase, indicating a stronger attraction to the polar silica gel.
More Information
In chromatography, a substance's affinity for the stationary phase is often determined by polarity. Polar substances adhere more strongly to polar phases, which slows their movement through the stationary phase.
Tips
A common mistake is assuming that a substance traveling farther has a stronger attraction to the stationary phase. In fact, it typically indicates weaker attraction.
Sources
- Separation of solutions and mixtures chromatography (practice) - khanacademy.org
- Principles of chromatography | Stationary phase (article) - khanacademy.org
AI-generated content may contain errors. Please verify critical information