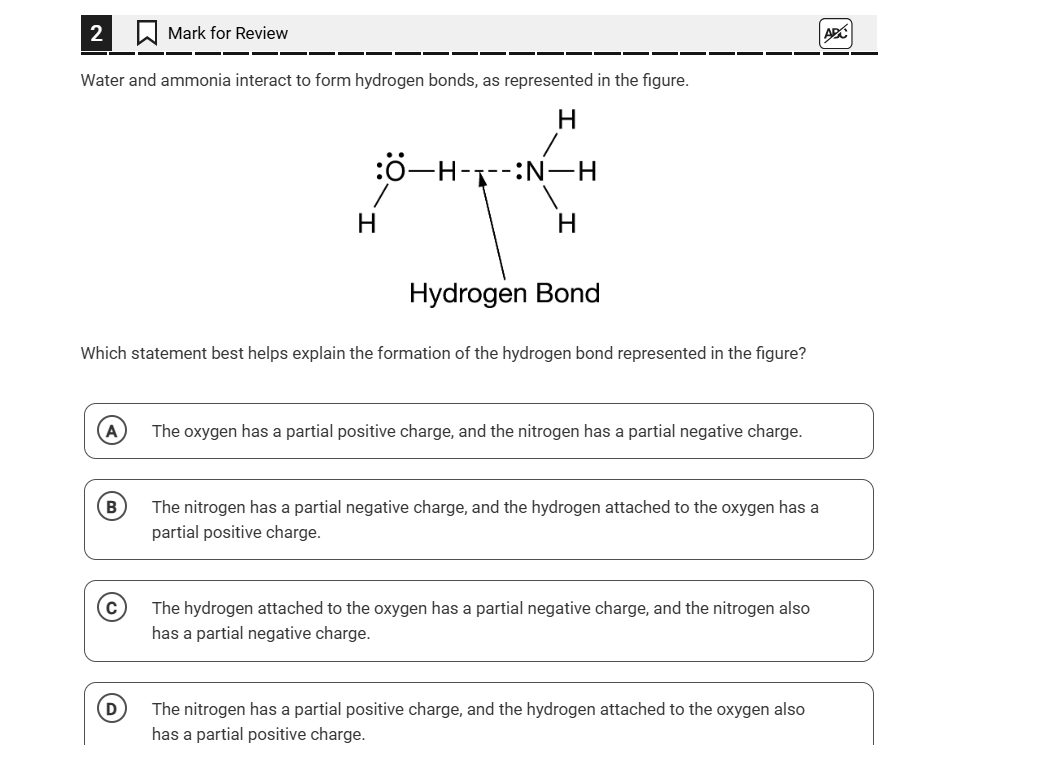
Which statement best helps explain the formation of the hydrogen bond represented in the figure?
Understand the Problem
The question is asking which statement accurately explains the formation of the hydrogen bond between water and ammonia, as indicated by the illustration provided. It requires an understanding of the partial charges associated with the atoms involved in the bond.
Answer
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
The statement that best helps explain the formation of the hydrogen bond represented in the figure is: The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
Answer for screen readers
The statement that best helps explain the formation of the hydrogen bond represented in the figure is: The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
More Information
Hydrogen bonds form when a hydrogen atom covalently bonded to an electronegative atom (like oxygen) experiences an attraction to another electronegative atom (like nitrogen) with a lone pair of electrons.
Tips
A common mistake is to confuse which atoms have partial positive and negative charges. Remember that hydrogen involved in hydrogen bonds is partially positive, and the electronegative atom it bonds with (like nitrogen or oxygen) is partially negative.
Sources
AI-generated content may contain errors. Please verify critical information