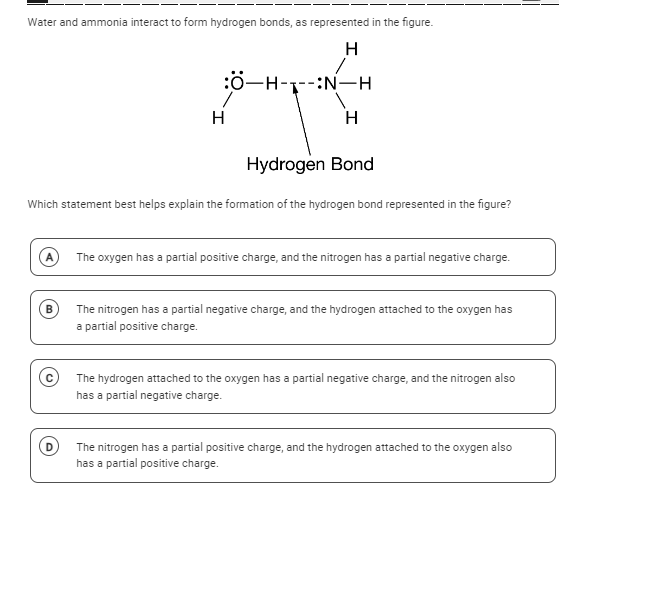
Which statement best helps explain the formation of the hydrogen bond represented in the figure?
Understand the Problem
The question is asking which statement accurately explains the formation of a hydrogen bond between water and ammonia as depicted in the provided diagram. It requires understanding the partial charges on oxygen and nitrogen atoms in the context of hydrogen bonding.
Answer
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
Answer for screen readers
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
More Information
Hydrogen bonds are electrostatic attractions between partially positive hydrogen atoms and electronegative atoms like nitrogen, oxygen, or fluorine with lone pairs.
Tips
A common mistake is not recognizing the partial charges due to electronegativity differences. Remember that hydrogen attached to highly electronegative atoms (like oxygen) will have a partial positive charge.