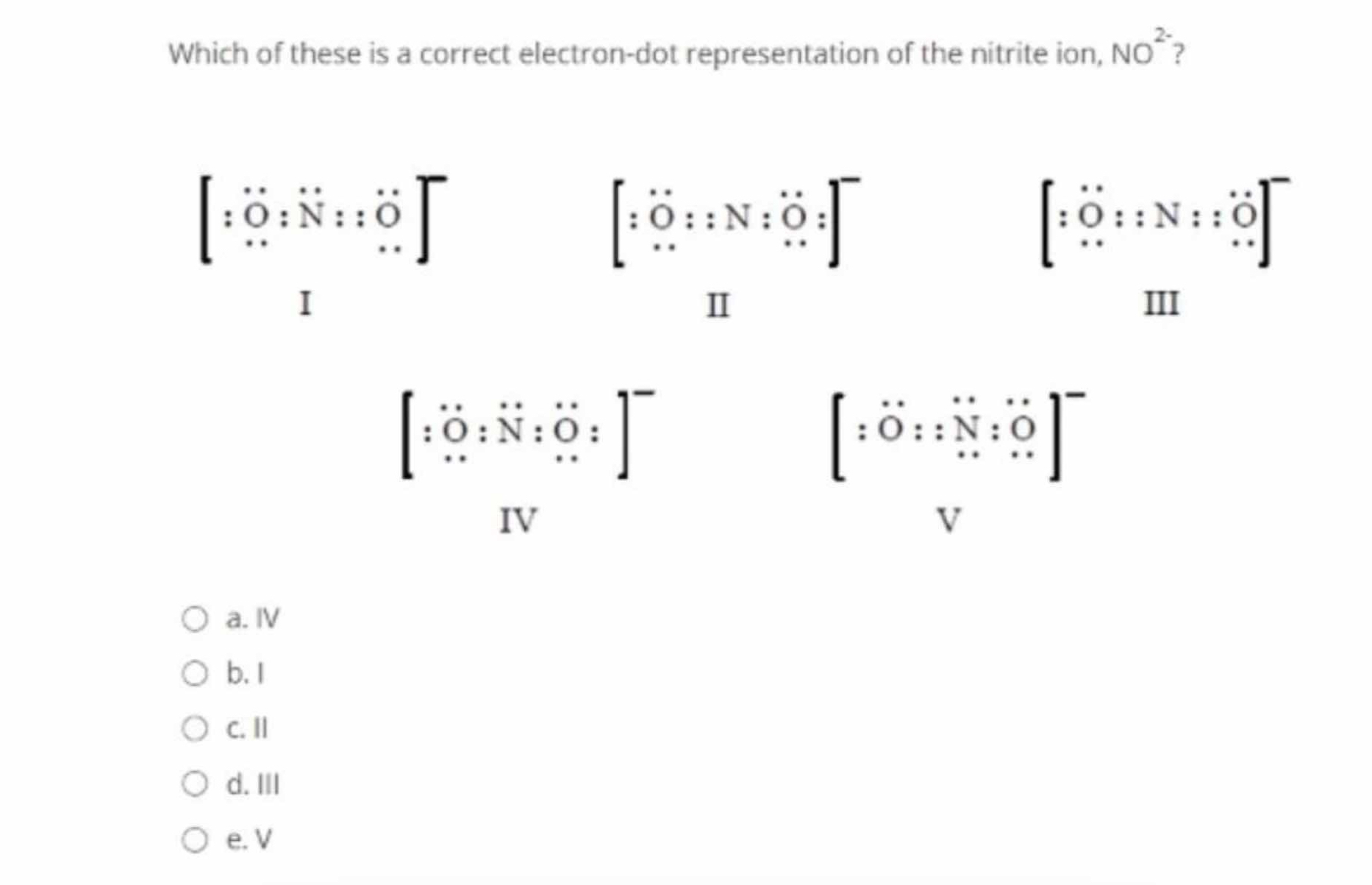
Which of these is a correct electron-dot representation of the nitrite ion, NO2-?
Understand the Problem
The question is asking which of the provided structures correctly represents the electron-dot configuration of the nitrite ion (NO2-). This involves understanding Lewis structures and the arrangement of electrons around the nitrogen and oxygen atoms.
Answer
IV
The correct electron-dot representation of the nitrite ion, NO2-, is IV.
Answer for screen readers
The correct electron-dot representation of the nitrite ion, NO2-, is IV.
More Information
In the correct Lewis structure for NO2-, one oxygen is double-bonded to nitrogen, and the other is single-bonded with an extra lone electron pair to account for the -1 charge.
Tips
A common mistake is not accounting for the formal charge correctly, resulting in the wrong placement of electrons or bonds.
Sources
- Lewis Structure for NO2- - Terpconnect - terpconnect.umd.edu
AI-generated content may contain errors. Please verify critical information