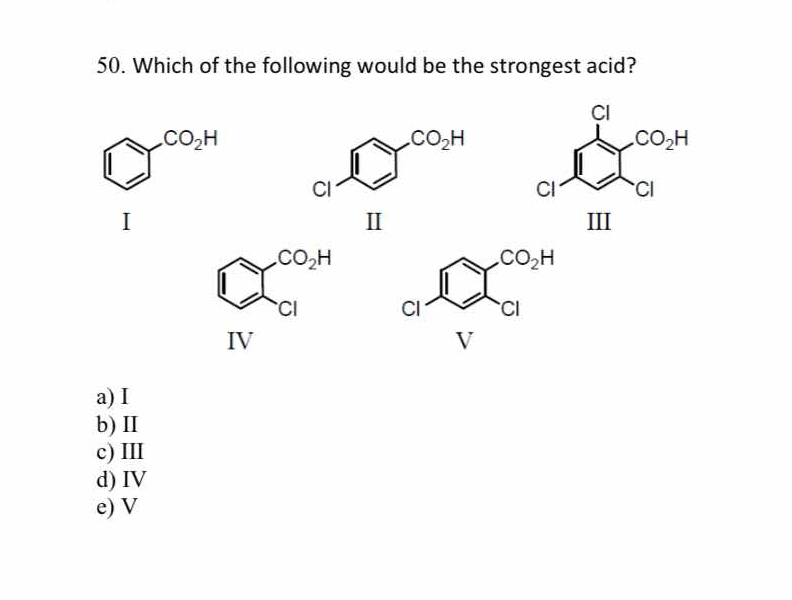
Which of the following would be the strongest acid?
Understand the Problem
The question is asking to identify which of the given structures represents the strongest acid. This involves analyzing the molecular structures, particularly the functional groups and substituents, to determine acidity.
Answer
III
The final answer is III.
Answer for screen readers
The final answer is III.
More Information
Compound III, with two chlorine atoms as electron-withdrawing groups in the ortho and para positions, increases the acidity by stabilizing the conjugate base more effectively than the others.
Tips
Common mistakes include not considering the position of electron-withdrawing groups and their effect on acidity.
AI-generated content may contain errors. Please verify critical information