Which of the following best describes the effect of a greater number of cysteine amino acids on the stability of the proteins?
Understand the Problem
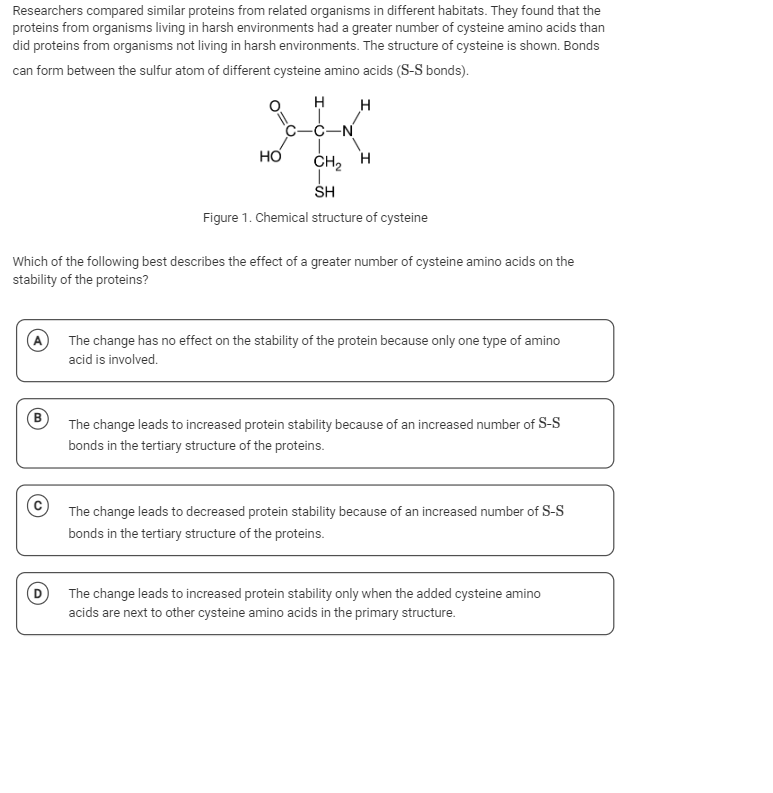
The question is asking for an explanation of how an increased number of cysteine amino acids affects protein stability, particularly in relation to the formation of S-S bonds in the protein's tertiary structure.
Answer
Increased protein stability because of more S-S bonds.
The change leads to increased protein stability because of an increased number of S-S bonds in the tertiary structure of the proteins.
Answer for screen readers
The change leads to increased protein stability because of an increased number of S-S bonds in the tertiary structure of the proteins.
More Information
Cysteine amino acids can form disulfide bonds which are covalent bonds that add significant stability to the folded protein structure.
Tips
A common mistake is to think that the presence of more cysteine automatically affects neighboring cysteines. It's the formation of disulfide bonds that is crucial for stability.
Sources
- Which of the following best describes the effect of a greater number of cysteine amino acids on the stability of the proteins? - coursesidekick.com
- Which of the following best describes the effect of a greater number ... - numerade.com
- please answer truly! SH Figure 1. Chemical structure of cysteine... - coursehero.com
AI-generated content may contain errors. Please verify critical information