Which of the following best describes how the structure of ice benefits the organisms that live in the water below?
Understand the Problem
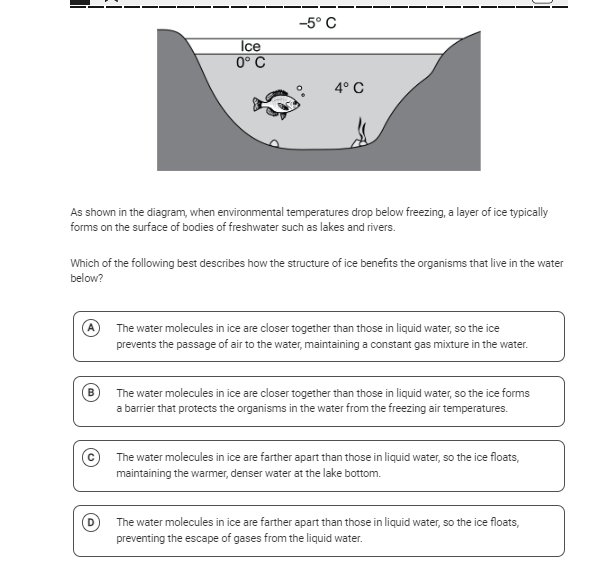
The question is asking how the structure of ice benefits aquatic organisms when temperatures drop below freezing, as illustrated in the diagram. It requires an understanding of the physical properties of ice compared to liquid water and its implications for freshwater ecosystems.
Answer
Ice floats because water molecules are farther apart, insulating warmer water below.
The final answer is that the water molecules in ice are farther apart than those in liquid water, so the ice floats, maintaining the warmer, denser water at the lake bottom.
Answer for screen readers
The final answer is that the water molecules in ice are farther apart than those in liquid water, so the ice floats, maintaining the warmer, denser water at the lake bottom.
More Information
Ice is less dense than liquid water, allowing it to float and form an insulating layer that protects aquatic life from cold air temperatures by keeping the layer of water beneath it slightly warmer.
Tips
A common mistake is to confuse the density properties of ice versus liquid water, leading to misconceptions about how ice forms a protective layer.
Sources
- 2.2 Water – Concepts of Biology – 1st Canadian Edition - opentextbc.ca
AI-generated content may contain errors. Please verify critical information