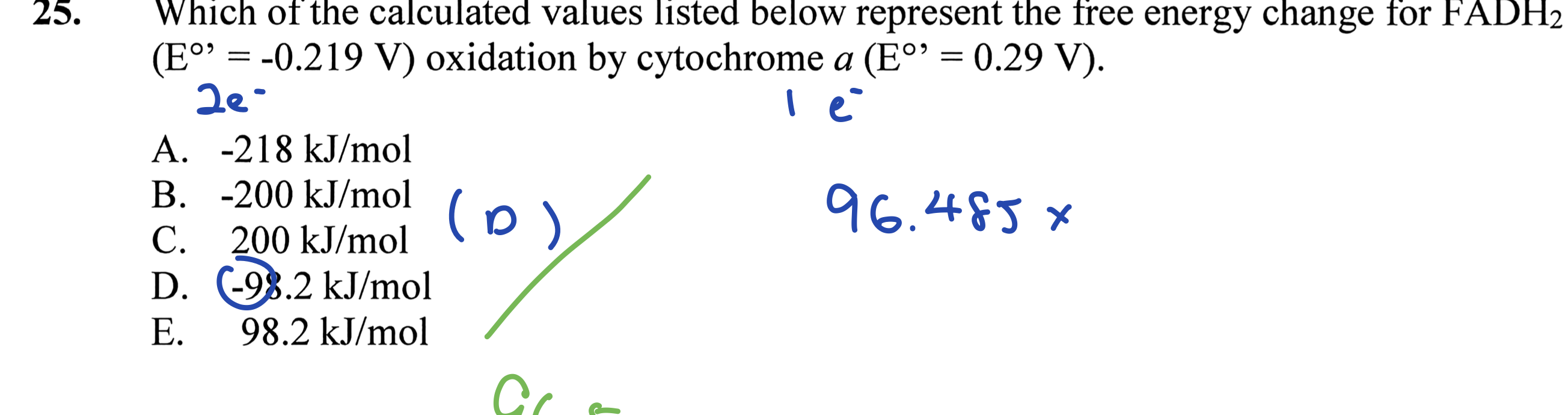
Which of the calculated values listed below represent the free energy change for FADH2 oxidation by cytochrome a?
Understand the Problem
The question is asking to identify the free energy change for FADH2 oxidation by cytochrome a using the provided standard reduction potentials (E°) and the formula to convert these potentials to free energy changes (ΔG). The user is expected to select the correct value from the options given.
Answer
-98.2 kJ/mol
The free energy change for FADH2 oxidation by cytochrome a is -98.2 kJ/mol.
Answer for screen readers
The free energy change for FADH2 oxidation by cytochrome a is -98.2 kJ/mol.
More Information
Oxidation involves transferring electrons from FADH2 to cytochrome a, releasing energy as indicated by the negative ΔG°'.
Tips
A common mistake is incorrect calculation of ΔE°' or the number of electrons, both of which affect ΔG°'.
Sources
- Oxidative Phosphorylation - an overview | ScienceDirect Topics - sciencedirect.com
AI-generated content may contain errors. Please verify critical information