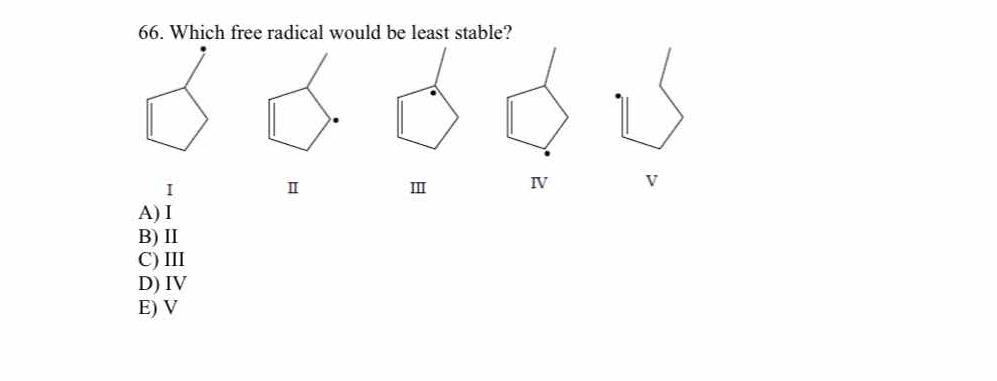
Which free radical would be least stable?
Understand the Problem
The question is asking which of the given free radicals, represented by structural formulas I through V, is the least stable. This involves understanding the stability of free radicals based on their structure and electronic configuration.
Answer
V
The final answer is V.
Answer for screen readers
The final answer is V.
More Information
Radical V is the least stable because it lacks resonance stabilization, unlike the other options.
Tips
A common mistake is not considering all forms of stabilization like resonance and hyperconjugation.
AI-generated content may contain errors. Please verify critical information