What is the relationship between the branching of organic molecules and their solubility in water, particularly in the case of isobutanol and butanol?
Understand the Problem
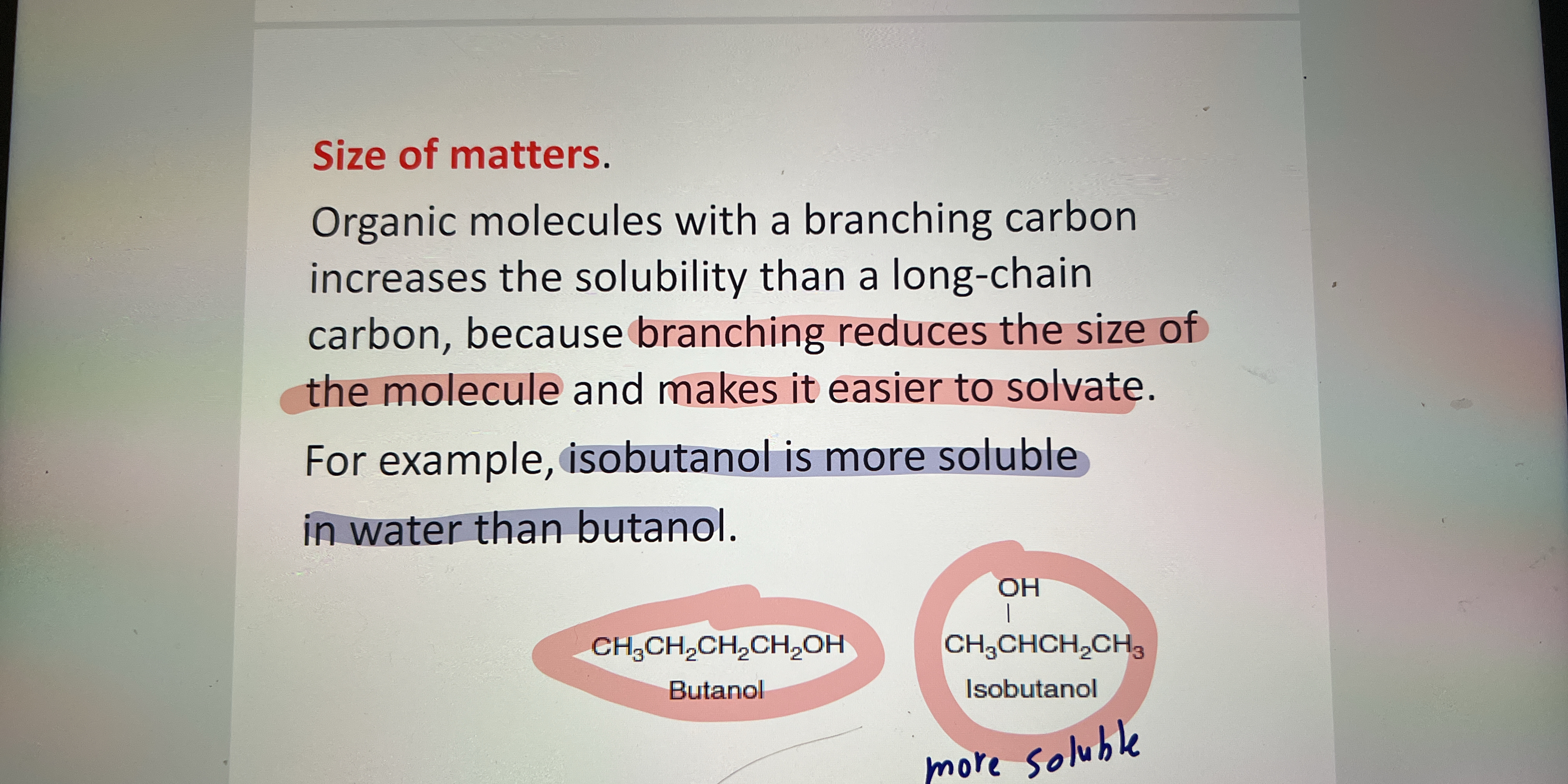
The question is discussing the solubility of organic molecules, specifically comparing branched versus unbranched carbon chains, with examples of isobutanol and butanol. It focuses on how branching affects solubility in water.
Answer
Isobutanol is more soluble in water than butanol because branching increases solubility.
The more branched isomer, isobutanol, is more soluble in water than butanol due to branching, which reduces hydrocarbon interactions and enhances the molecule's ability to interact with water.
Answer for screen readers
The more branched isomer, isobutanol, is more soluble in water than butanol due to branching, which reduces hydrocarbon interactions and enhances the molecule's ability to interact with water.
More Information
Branching in organic molecules reduces the extent of hydrophobic interactions among alkyl chains, making them more compatible with polar solvents like water. This is because branching increases the molecule's surface area in contact with the solvent, thus enhancing solubility.
Tips
A common mistake is assuming that more carbon means higher solubility in water, but it's the distribution and structure (branching) that matter more.
Sources
AI-generated content may contain errors. Please verify critical information