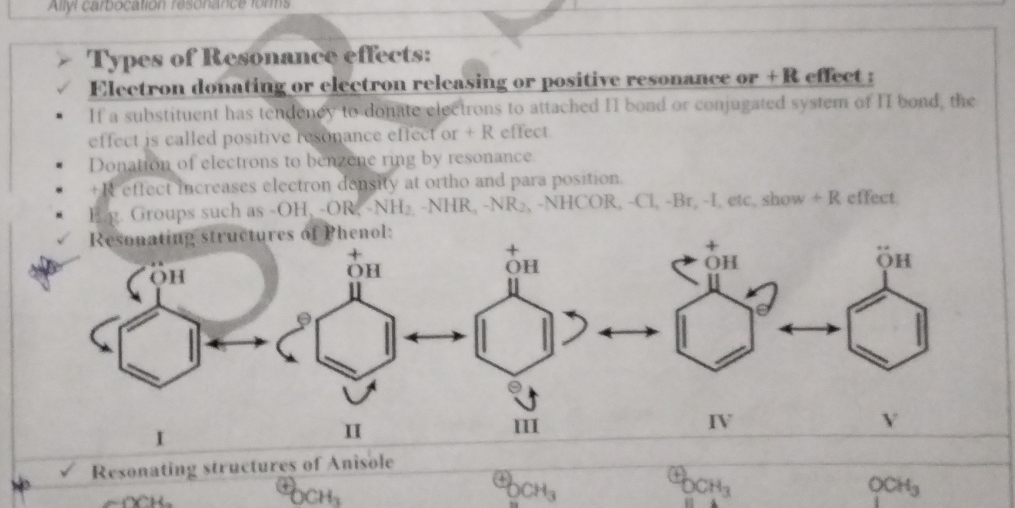
What are the types of resonance effects and the resonating structures of phenol?
Understand the Problem
The document discusses types of resonance effects, specifically the positive resonance effect involving electron-donating or electron-releasing substituents in compounds like phenol and anisol. It also illustrates the resonating structures for these compounds.
Answer
+R effect; five resonating structures for phenol.
The types of resonance effects are +R effect (positive resonance effect). In phenol, there are five resonating structures resulting from the delocalization of electrons from the oxygen atom to the benzene ring, stabilizing the structure.
Answer for screen readers
The types of resonance effects are +R effect (positive resonance effect). In phenol, there are five resonating structures resulting from the delocalization of electrons from the oxygen atom to the benzene ring, stabilizing the structure.
More Information
The +R effect in phenol occurs due to the electron-donating group (-OH) which increases electron density within the aromatic ring.
Tips
Ensure correct direction of electron flow with arrows in resonance structures.
Sources
AI-generated content may contain errors. Please verify critical information