What is the product of the reaction between CH3-CH2-CH2-COOH and NaOH?
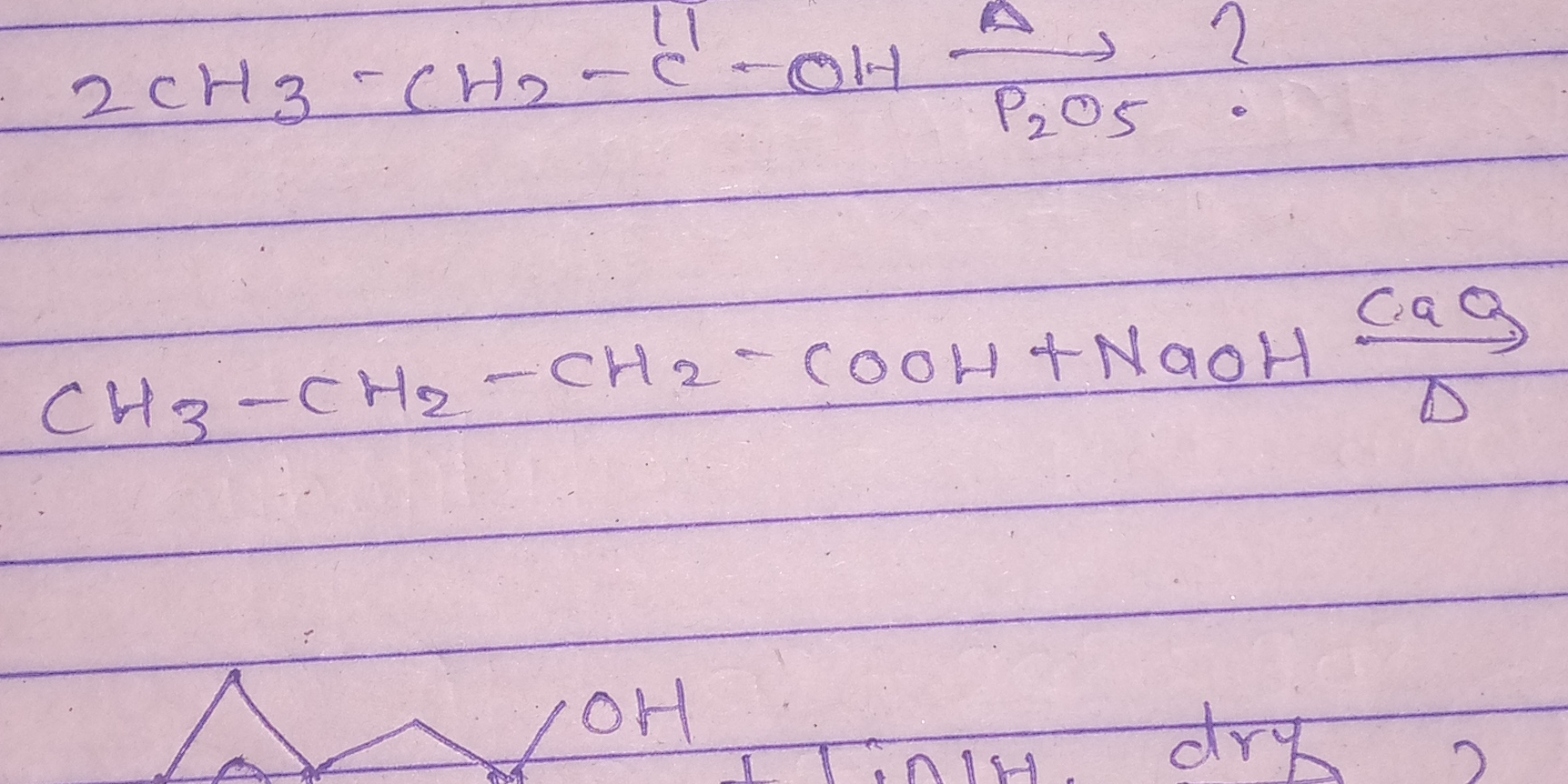
Understand the Problem
The question appears to involve organic chemistry reactions, specifically looking at the reaction of a compound with NaOH, possibly in the context of a synthesis or reaction mechanism. The user seems to be asking for the product of this reaction.
Answer
The product is butanoic acid ($CH_3-CH_2-CH_2-COOH$).
Answer for screen readers
The product of the reaction is butanoic acid ($CH_3-CH_2-CH_2-COOH$).
Steps to Solve
-
Identifying the Reactants and Reaction Conditions
The reactants given are two molecules of 2-butanol ($CH_3-CH_2-CH(OH)-CH_3$) and phosphorus pentoxide ($P_2O_5$), with NaOH as a base, indicating this reaction may follow dehydration and base-induced processes.
-
Dehydration of Alcohol
The first step involves the dehydration of 2-butanol, which occurs when treated with $P_2O_5$. Dehydration results in the formation of butene ($CH_3-CH_2-CH=CH_2$) and water.
-
Base-Induced Reaction
Now, when butene reacts with NaOH, it can undergo a hydrolysis reaction under alkaline conditions, forming butanol. The complete reaction from the starting alcohol to the product can be represented as:
$$ 2 CH_3-CH_2-CH(OH)-CH_3 \xrightarrow{P_2O_5} CH_3-CH_2-CH=CH_2 + H_2O $$
-
Formation of Carboxylic Acid
The butene can further react (upon oxidation) to yield butanoic acid ($CH_3-CH_2-CH_2-COOH$), summarized by:
$$ CH_3-CH_2-CH=CH_2 \xrightarrow{oxidation} CH_3-CH_2-CH_2-COOH $$
-
Final Products in the Reaction
The complete reaction leads to butanoic acid as the final product, with water as a byproduct from the dehydration stage.
The product of the reaction is butanoic acid ($CH_3-CH_2-CH_2-COOH$).
More Information
This reaction highlights the alcohol's transformation through dehydration and subsequent hydrolysis, typical in organic synthesis. The use of $P_2O_5$ indicates a dehydration catalyst, which is a common practice in organic reactions to facilitate alcohol conversion.
Tips
- Misidentifying the products of dehydration: Always remember that dehydration will yield alkenes, not alcohols.
- Forgetting to consider the need for oxidation if a carboxylic acid is the desired product from an alkene.
AI-generated content may contain errors. Please verify critical information