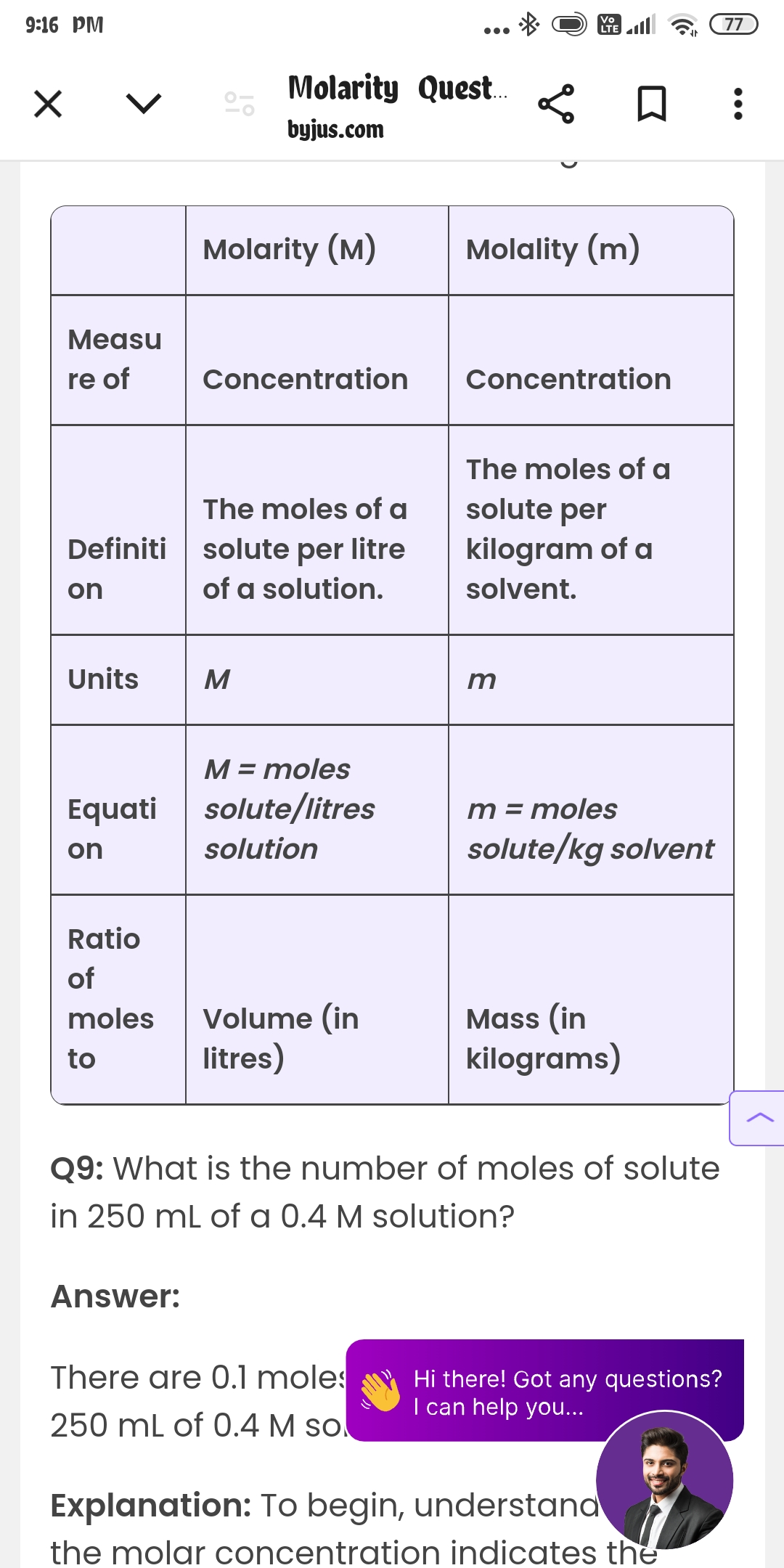
What is the number of moles of solute in 250 mL of a 0.4 M solution?
Understand the Problem
The question is asking for the number of moles of solute in a given volume (250 mL) of a solution with a specified concentration (0.4 M). This involves using the formula that relates molarity to moles and volume.
Answer
The number of moles of solute is $0.1$ moles.
Answer for screen readers
The number of moles of solute in 250 mL of a 0.4 M solution is $0.1$ moles.
Steps to Solve
- Convert Volume to Liters
First, convert the volume from milliliters (mL) to liters (L).
Since 1 L = 1000 mL, then: $$ \text{Volume in L} = \frac{250 \text{ mL}}{1000} = 0.25 \text{ L} $$
- Use the Molarity Formula
Next, use the molarity formula to determine the number of moles of solute. The formula is given by: $$ M = \frac{\text{moles of solute}}{\text{liters of solution}} $$ Rearranging gives: $$ \text{moles of solute} = M \times \text{liters of solution} $$
- Substitute Values
Now, substitute the values into the formula: $$ \text{moles of solute} = 0.4 \text{ M} \times 0.25 \text{ L} $$
- Calculate the Moles
Now calculate the moles of solute: $$ \text{moles of solute} = 0.4 \times 0.25 = 0.1 \text{ moles} $$
The number of moles of solute in 250 mL of a 0.4 M solution is $0.1$ moles.
More Information
In solutions, molarity (M) is commonly used to express concentration. It tells how many moles of solute are present per liter of solution. This calculation is essential in chemistry for preparing solutions and performing reactions.
Tips
- Converting mL to L incorrectly: Remember that 1000 mL = 1 L.
- Forgetting to multiply the molarity by the correct volume in liters.
- Misunderstanding the relationship between moles, molarity, and volume.
AI-generated content may contain errors. Please verify critical information