What is the definition of equilibrium in chemistry?
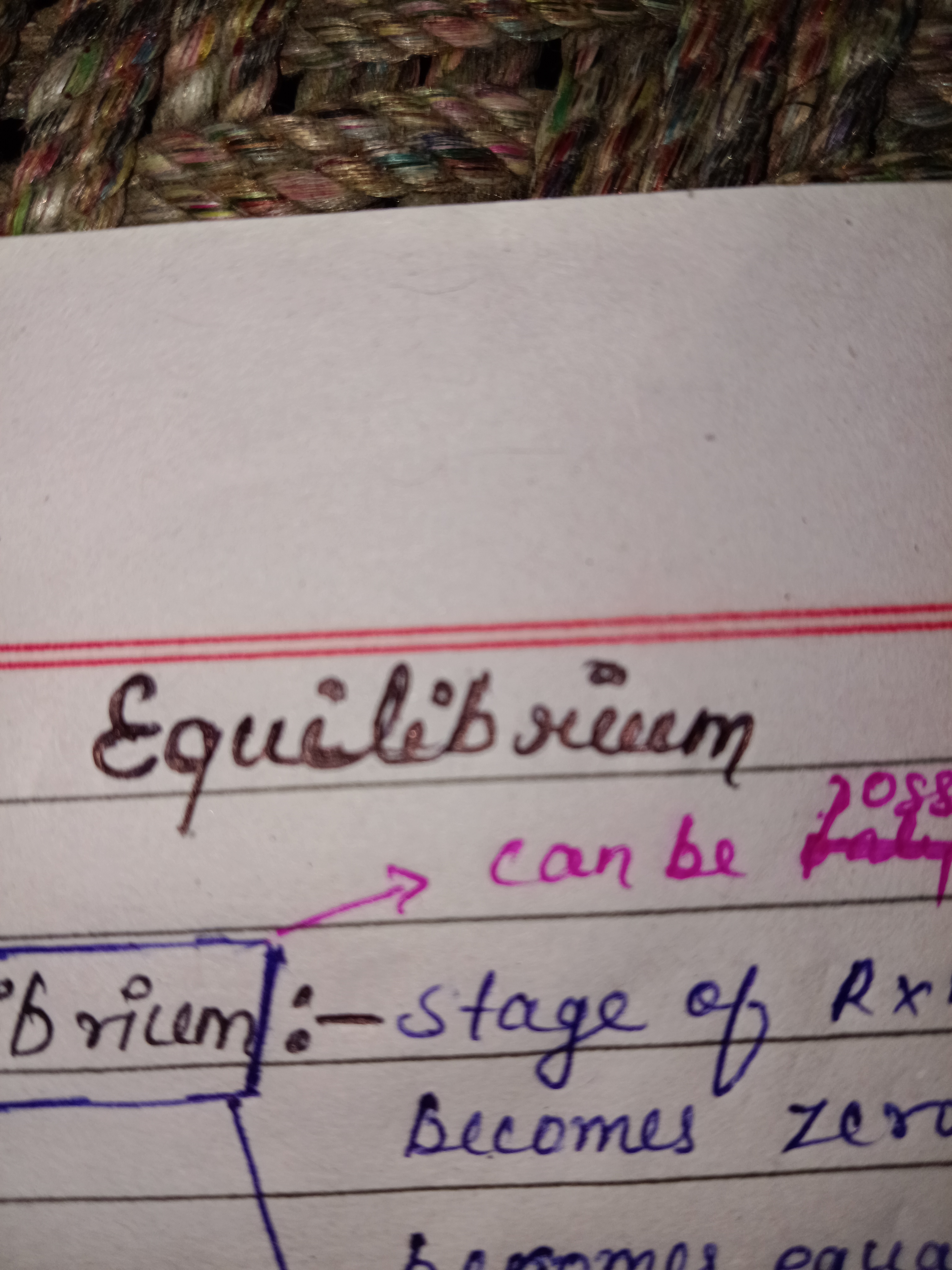
Understand the Problem
The content in the image appears to be discussing the concept of equilibrium in a scientific context, possibly related to reactions or states of matter.
Answer
Equilibrium is when forward and reverse reaction rates are equal.
In chemistry, equilibrium is the state of a reversible reaction where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time.
Answer for screen readers
In chemistry, equilibrium is the state of a reversible reaction where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time.
More Information
At equilibrium, there is a dynamic balance with ongoing reactions at a molecular level, but no overall concentration changes in the system.
Tips
A common mistake is thinking that reactions stop at equilibrium. They continue but do not change concentrations.
Sources
- Chemical equilibrium | Definition, Equation, & Facts - Britannica - britannica.com
- Principles of Chemical Equilibrium - Chemistry LibreTexts - chem.libretexts.org
AI-generated content may contain errors. Please verify critical information