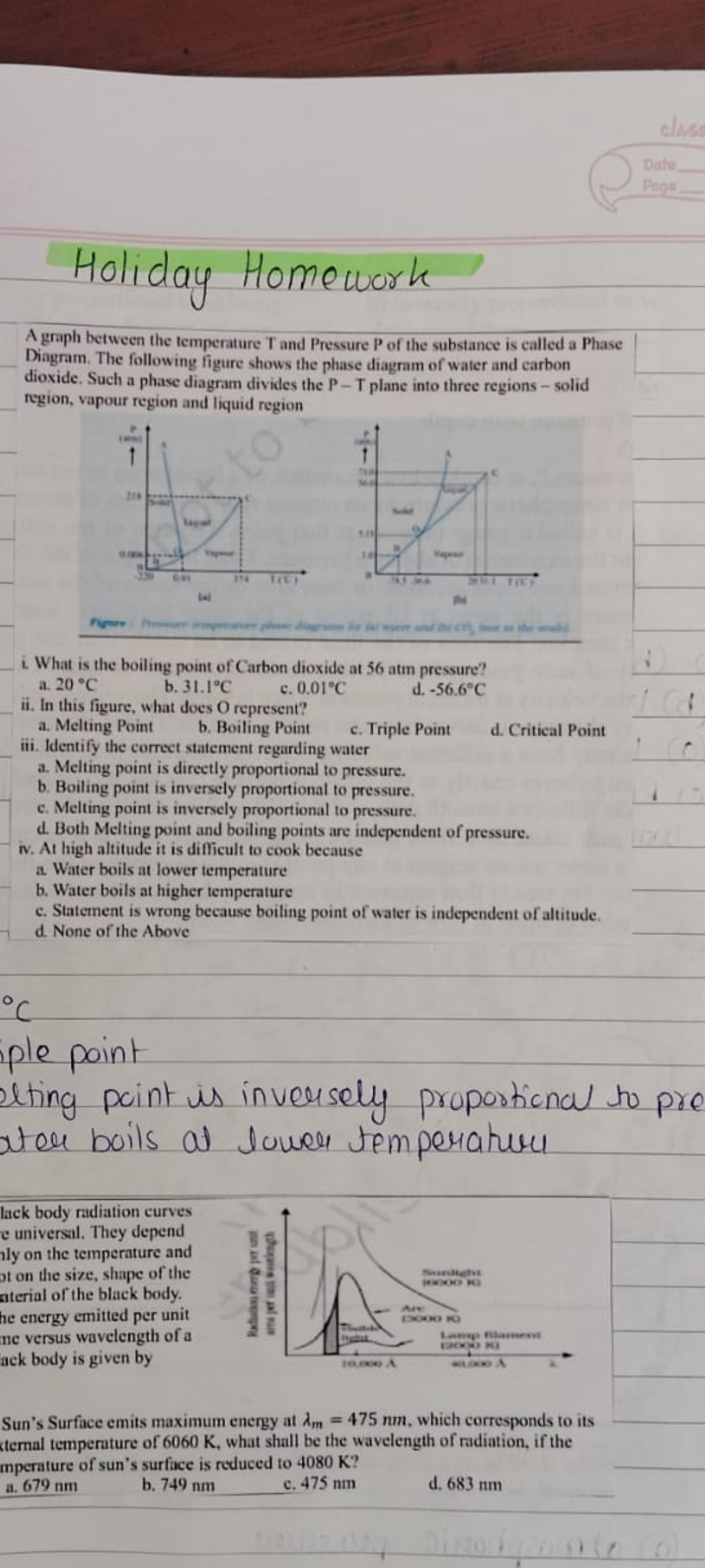
What is the boiling point of Carbon dioxide at 56 atm pressure? In this figure, what does O represent? Identify the correct statement regarding water: At high altitude it is diffic... What is the boiling point of Carbon dioxide at 56 atm pressure? In this figure, what does O represent? Identify the correct statement regarding water: At high altitude it is difficult to cook because.
Understand the Problem
The question is asking about the boiling point of carbon dioxide at a specific pressure and involves understanding phase diagrams and principles of thermodynamics. It also includes additional questions related to properties of water and the relationship between temperature and pressure regarding boiling points.
Answer
Boiling point: 31.1°C. O: Triple point. Water boils at lower temperature at high altitude.
The boiling point of carbon dioxide at 56 atm is 31.1°C. In the figure, O represents the triple point. At high altitudes, it is difficult to cook because water boils at a lower temperature.
Answer for screen readers
The boiling point of carbon dioxide at 56 atm is 31.1°C. In the figure, O represents the triple point. At high altitudes, it is difficult to cook because water boils at a lower temperature.
More Information
At high altitudes, lower atmospheric pressure results in water boiling at temperatures below 100°C, making cooking slower.
Tips
A common mistake is assuming water boils at 100°C regardless of altitude due to misunderstanding pressure effects.
AI-generated content may contain errors. Please verify critical information