What are the states of matter and their characteristics? Include information on changes in state and examples.
Understand the Problem
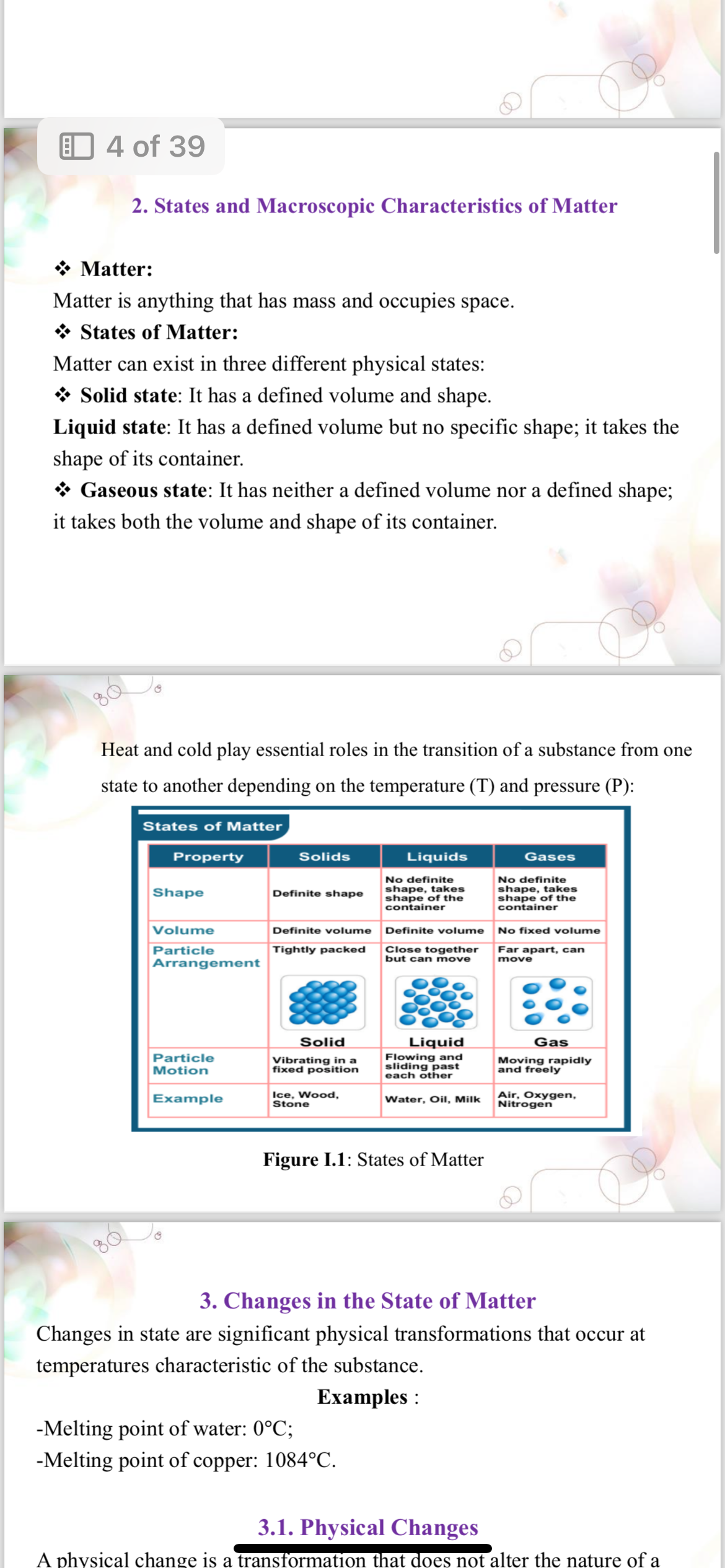
The question provides information about the states of matter and their characteristics, as well as changes in the state of matter, specifically focusing on physical transformations and examples of melting points of substances.
Answer
Solid, liquid, gas, plasma are states of matter, with changes like melting and evaporation.
The main states of matter are solid, liquid, gas, and plasma. Solids have definite shape and volume; liquids have definite volume but no shape; gases have neither; plasma consists of free ions and electrons. Changes in state occur through processes like melting and evaporation.
Answer for screen readers
The main states of matter are solid, liquid, gas, and plasma. Solids have definite shape and volume; liquids have definite volume but no shape; gases have neither; plasma consists of free ions and electrons. Changes in state occur through processes like melting and evaporation.
More Information
The four main states represent the most common forms of matter in the universe. Transitioning between these states involves energy changes, typically heating or cooling. Other less common states include Bose-Einstein condensate and time crystals.
Tips
Common mistakes include confusing the properties of solids, liquids, and gases, especially regarding shape and volume. Remember that temperature and pressure influence state changes.
Sources
- States of Matter | Characteristics, Comparison & Examples - Study.com - study.com
- 8.1: States of Matter and Their Changes - Chemistry LibreTexts - chem.libretexts.org
- States of matter: Definition and phases of change | Live Science - livescience.com
AI-generated content may contain errors. Please verify critical information