What are the properties of water and how do they benefit life, along with the transport of metabolites in the blood?
Understand the Problem
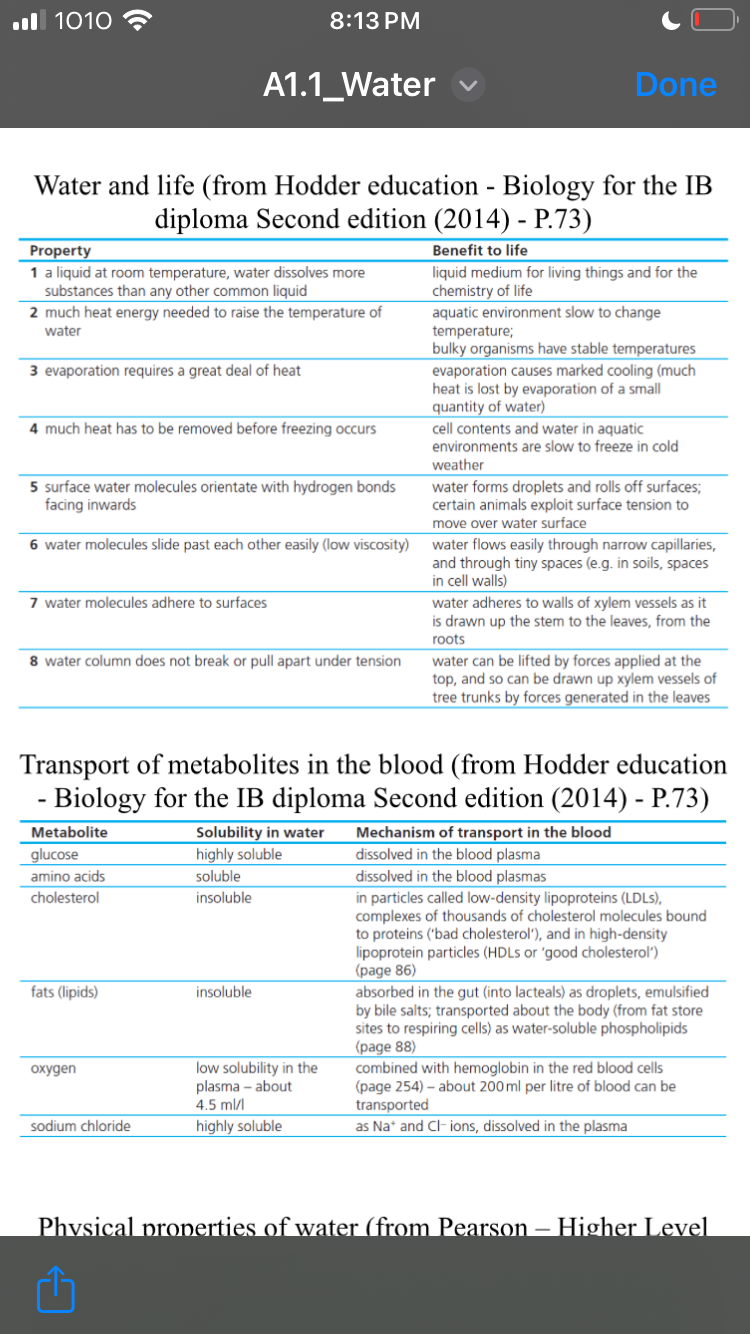
The question involves understanding the physical and chemical properties of water, particularly how these properties benefit life forms and the transport of metabolites in the blood. It requires an analysis of two tables summarizing essential information about water related to biology.
Answer
Water dissolves nutrients, stabilizes temperature, supports transport in blood. Cholesterol and fats need carriers.
Water supports life through its properties: it's a universal solvent facilitating biochemical reactions; its cohesion and adhesion enable nutrient transport. Thermal properties stabilize environments. In blood, water dissolves and transports metabolites like glucose and oxygen, while cholesterol and fats form micelles for transport.
Answer for screen readers
Water supports life through its properties: it's a universal solvent facilitating biochemical reactions; its cohesion and adhesion enable nutrient transport. Thermal properties stabilize environments. In blood, water dissolves and transports metabolites like glucose and oxygen, while cholesterol and fats form micelles for transport.
More Information
Water’s polarity allows it to dissolve various substances, making it essential for cellular reactions. Its thermal stability helps maintain consistent environmental temperatures crucial for homeostasis.
Tips
Don't confuse cohesion and adhesion; cohesion is water binding to itself, adhesion is binding to other substances.
Sources
- Lesson summary: Water and life (article) | Khan Academy - khanacademy.org
- Biological Roles of Water: Why is water necessary for life? - sitn.hms.harvard.edu
AI-generated content may contain errors. Please verify critical information