What are the properties and examples of acids and bases?
Understand the Problem
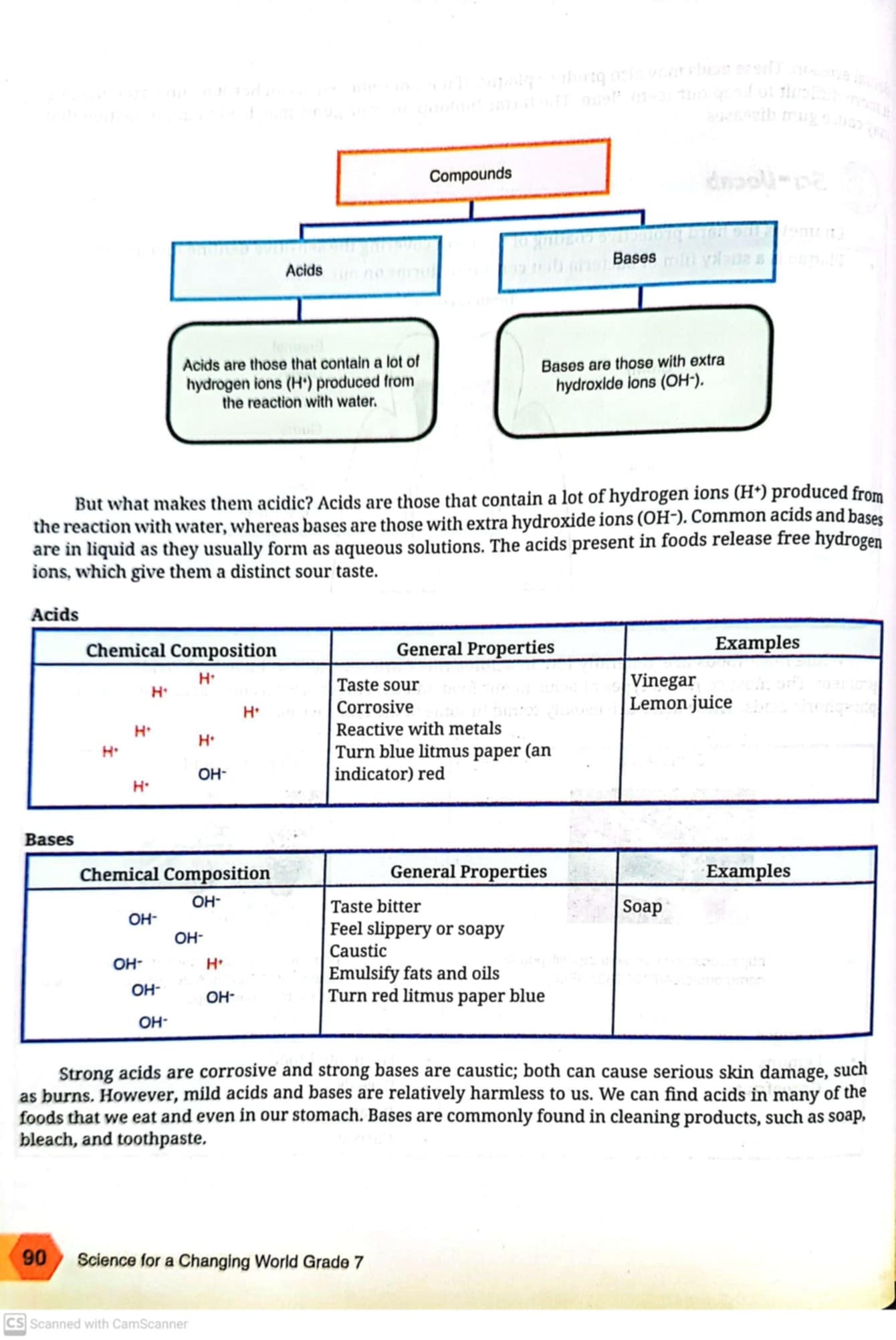
The question is examining the characteristics of acids and bases, specifically their chemical composition, properties, and examples. It explains how acids produce hydrogen ions and how bases produce hydroxide ions, highlighting their different tastes and reactions.
Answer
Acids: H+, sour, corrosive, turn blue litmus red, e.g., vinegar, lemon juice. Bases: OH-, bitter, slippery, turn red litmus blue, e.g., soap.
Acids are substances that contain hydrogen ions (H+) and have properties such as sour taste, corrosiveness, and the ability to turn blue litmus paper red. Examples include vinegar and lemon juice. Bases contain hydroxide ions (OH-) and have properties such as bitter taste, slippery feel, and the ability to turn red litmus paper blue. Examples include soap.
Answer for screen readers
Acids are substances that contain hydrogen ions (H+) and have properties such as sour taste, corrosiveness, and the ability to turn blue litmus paper red. Examples include vinegar and lemon juice. Bases contain hydroxide ions (OH-) and have properties such as bitter taste, slippery feel, and the ability to turn red litmus paper blue. Examples include soap.
More Information
Acids and bases play crucial roles in chemistry and everyday life. Acids, such as vinegar and lemon juice, are frequently found in foods, while bases, like soap and bleach, are common in cleaning products.
Tips
A common mistake is confusing the pH ranges: acids have a pH less than 7, while bases have a pH greater than 7. Remember that acids turn blue litmus paper red, and bases turn red litmus paper blue.
Sources
- Difference between Acids and Bases - byjus.com
- Properties of Acids and Bases - ChemTalk - chemistrytalk.org
- Properties of Acids and Bases - Physical and Chemical - BYJU'S - byjus.com
AI-generated content may contain errors. Please verify critical information