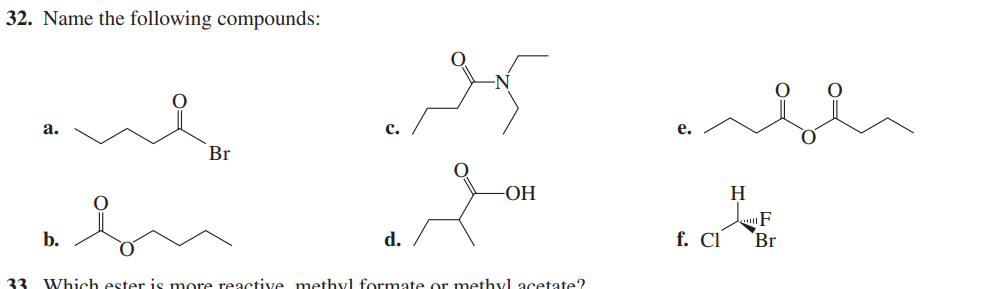
Name the following compounds: a. b. c. d. e. f. Which ester is more reactive, methyl formate or methyl acetate?
Understand the Problem
The question is asking for the names of several organic compounds displayed in a diagram. It is also inquiring which ester, methyl formate or methyl acetate, is more reactive. To solve this, we will identify the compounds represented in the image and discuss the reactivity of the listed esters.
Answer
a. 2-Bromobutanone, b. Butyl acetate, c. N,N-Diisopropylbutanamide, d. 3-Hydroxybutanoic acid, e. Diethyl malonate, f. 2-Bromo-2-chloro-1-fluoropropane. Methyl formate is more reactive.
The names of the compounds are: a. 2-Bromobutanone, b. Butyl acetate, c. N,N-Diisopropylbutanamide, d. 3-Hydroxybutanoic acid, e. Diethyl malonate, f. 2-Bromo-2-chloro-1-fluoropropane. Methyl formate is more reactive than methyl acetate.
Answer for screen readers
The names of the compounds are: a. 2-Bromobutanone, b. Butyl acetate, c. N,N-Diisopropylbutanamide, d. 3-Hydroxybutanoic acid, e. Diethyl malonate, f. 2-Bromo-2-chloro-1-fluoropropane. Methyl formate is more reactive than methyl acetate.
More Information
Methyl formate's increased reactivity is due to the lack of alkyl electron-donating groups. This raises the partial positive charge on the carbonyl carbon and enhances its reactivity.
Tips
Watch out for possible confusing elements in compound structures, like the position of substituents in esters or amines.
Sources
- Reactive Esters Explanation - Quizlet - quizlet.com
AI-generated content may contain errors. Please verify critical information