What are the methods for separating compounds and mixtures in chemistry, and what principles do they rely on?
Understand the Problem
The question involves understanding key concepts and methods in chemistry for separating mixtures and elements. It outlines processes such as filtration, distillation, and chromatography, as well as principles of mass conservation.
Answer
Compounds need chemical changes; mixtures use physical methods like filtration, distillation, and chromatography.
Common methods for separating mixtures include filtration (based on particle size), distillation (boiling points), and chromatography (polarity). Compounds require chemical changes to separate into elements.
Answer for screen readers
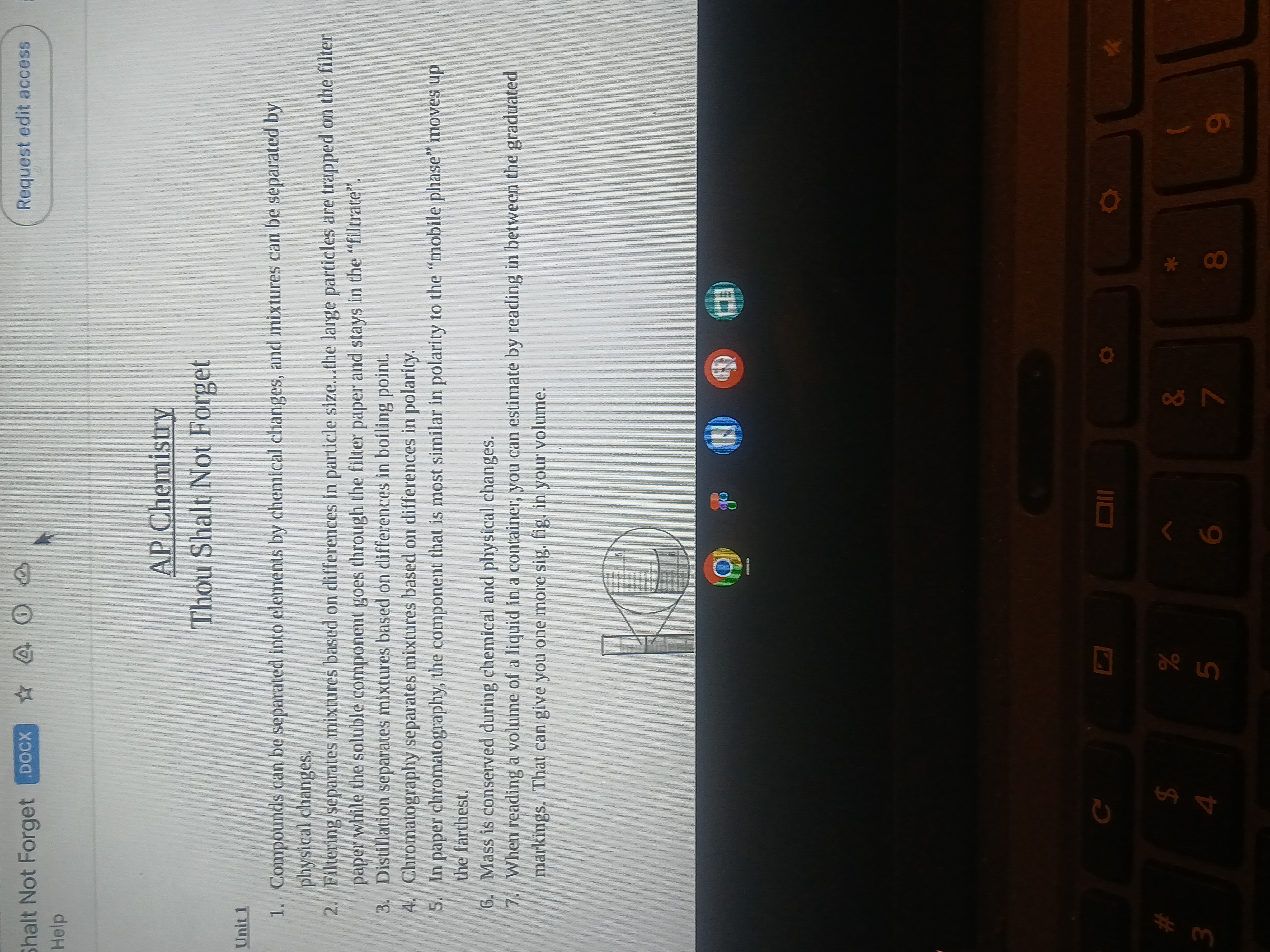
Common methods for separating mixtures include filtration (based on particle size), distillation (boiling points), and chromatography (polarity). Compounds require chemical changes to separate into elements.
More Information
Separation techniques rely on fundamental physical properties, allowing efficient extraction of components without altering chemical structures.
Tips
Ensure to match the separation technique with the property that most differs between the mixture's components.
Sources
- 1.4 Laboratory Techniques for Separation of Mixtures – CHEM 1114 - pressbooks.bccampus.ca
- Chemical Separations: Techniques & Methods | Vaia - vaia.com
AI-generated content may contain errors. Please verify critical information