What are the differences between physical change and chemical change?
Understand the Problem
The question is about distinguishing between physical and chemical changes. It outlines the characteristics of each type, such as the effects on substances, reversibility, and whether a new substance is formed.
Answer
Physical changes are reversible and don’t form new substances; chemical changes are irreversible and do form new substances.
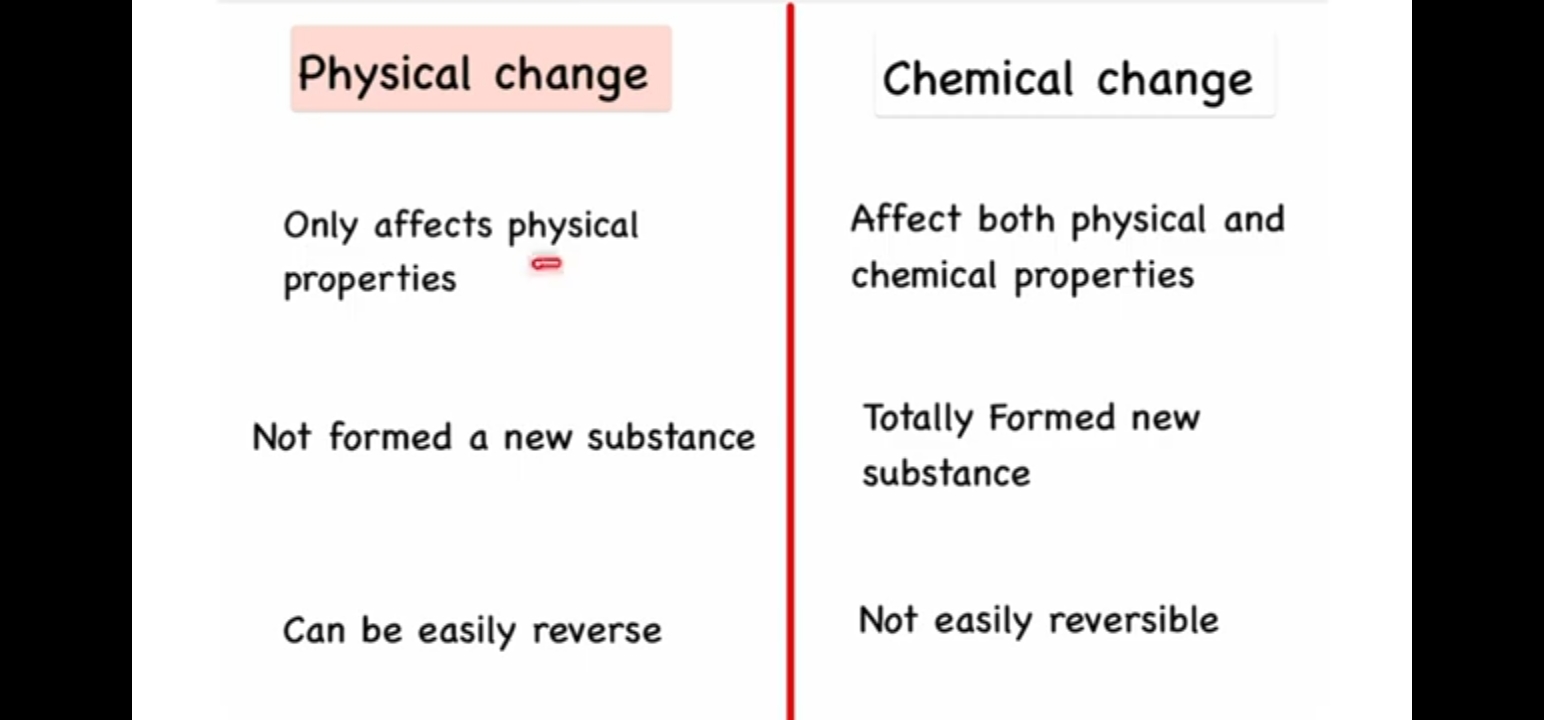
Physical changes affect only physical properties and do not form a new substance, making them easily reversible. Chemical changes affect both physical and chemical properties, producing a new substance, and are not easily reversible.
Answer for screen readers
Physical changes affect only physical properties and do not form a new substance, making them easily reversible. Chemical changes affect both physical and chemical properties, producing a new substance, and are not easily reversible.
More Information
Physical changes involve states or shapes, such as melting or cutting, while chemical changes involve reactions, resulting in new substances.
Tips
A common mistake is confusing dissolving with a chemical change; it's often a physical change unless a reaction occurs.
Sources
- Difference Between Physical And Chemical Change With Examples - byjus.com
- Chemical Change vs. Physical Change - Chemistry LibreTexts - chem.libretexts.org
- Changes in Matter: Physical vs. Chemical Changes - education.nationalgeographic.org
AI-generated content may contain errors. Please verify critical information