What are the differences between elements, compounds, and mixtures?
Understand the Problem
The question involves concepts related to elements, compounds, and mixtures, explaining their distinct characteristics and how they differ from each other.
Answer
Elements: one type of atom. Compounds: chemically bonded elements. Mixtures: substances physically mixed.
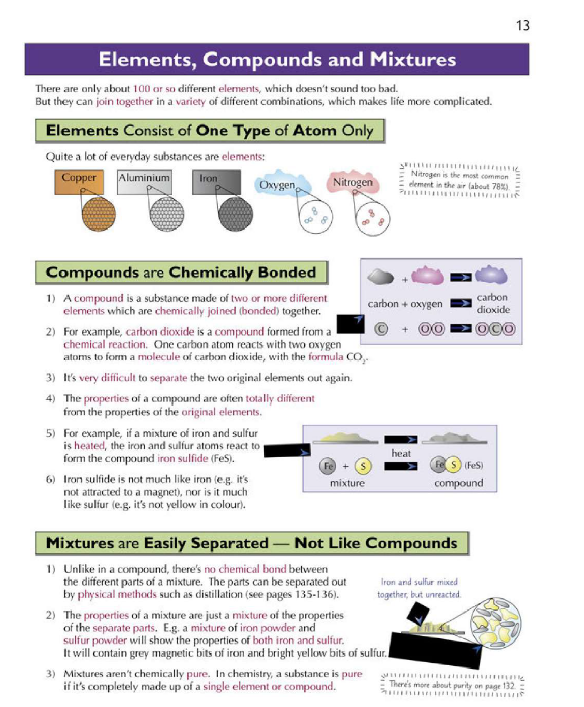
Elements consist of one type of atom. Compounds are substances made from two or more elements chemically bonded. Mixtures contain two or more substances not chemically bonded and can be separated physically.
Answer for screen readers
Elements consist of one type of atom. Compounds are substances made from two or more elements chemically bonded. Mixtures contain two or more substances not chemically bonded and can be separated physically.
More Information
Elements are pure substances with identical atoms. Compounds are pure but with atoms of different elements bonded. Mixtures are a combination of substances not chemically bonded, retaining original properties.
Tips
A common mistake is confusing mixtures and compounds. Remember, mixtures are physically combined and can be separated easily, unlike compounds.
Sources
- Elements, Compounds and Mixtures - RSC Education - edu.rsc.org
- Elements, Compounds & Mixtures - BBC Bitesize - bbc.co.uk
- Elements VS Compounds VS Mixtures - enthu.com
AI-generated content may contain errors. Please verify critical information