What are the common cations and anions in chemistry?
Understand the Problem
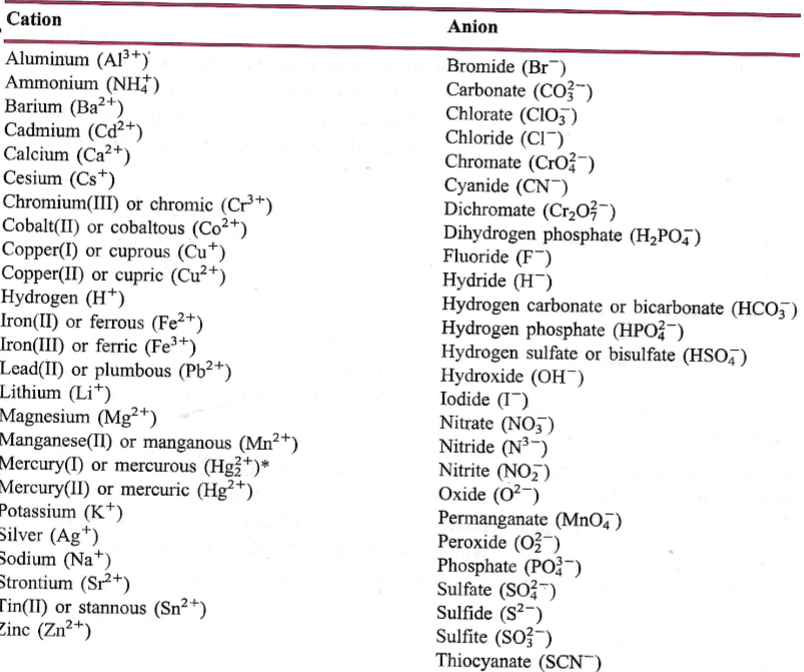
The question provides a table of cations and anions, likely related to ionic compounds in chemistry. It helps identify common ions used in chemical formulas.
Answer
Common cations: Na+, Ca2+, K+, NH4+. Common anions: Cl-, SO4^2-, NO3-, CO3^2-.
Common cations include sodium (Na+), calcium (Ca2+), potassium (K+), and ammonium (NH4+). Common anions include chloride (Cl-), sulfate (SO4^2-), nitrate (NO3-), and carbonate (CO3^2-).
Answer for screen readers
Common cations include sodium (Na+), calcium (Ca2+), potassium (K+), and ammonium (NH4+). Common anions include chloride (Cl-), sulfate (SO4^2-), nitrate (NO3-), and carbonate (CO3^2-).
More Information
Cations usually originate from metals and have a positive charge because they lose electrons. Anions typically come from nonmetals and have a negative charge because they gain electrons.
Tips
A common mistake is confusing cation and anion charges. Remember, cations are positive and anions are negative.
Sources
- Cation vs Anion: Definition, Chart and the Periodic Table - technologynetworks.com
- Chemistry Ions and Cations - Roy Mech - roymech.org
AI-generated content may contain errors. Please verify critical information