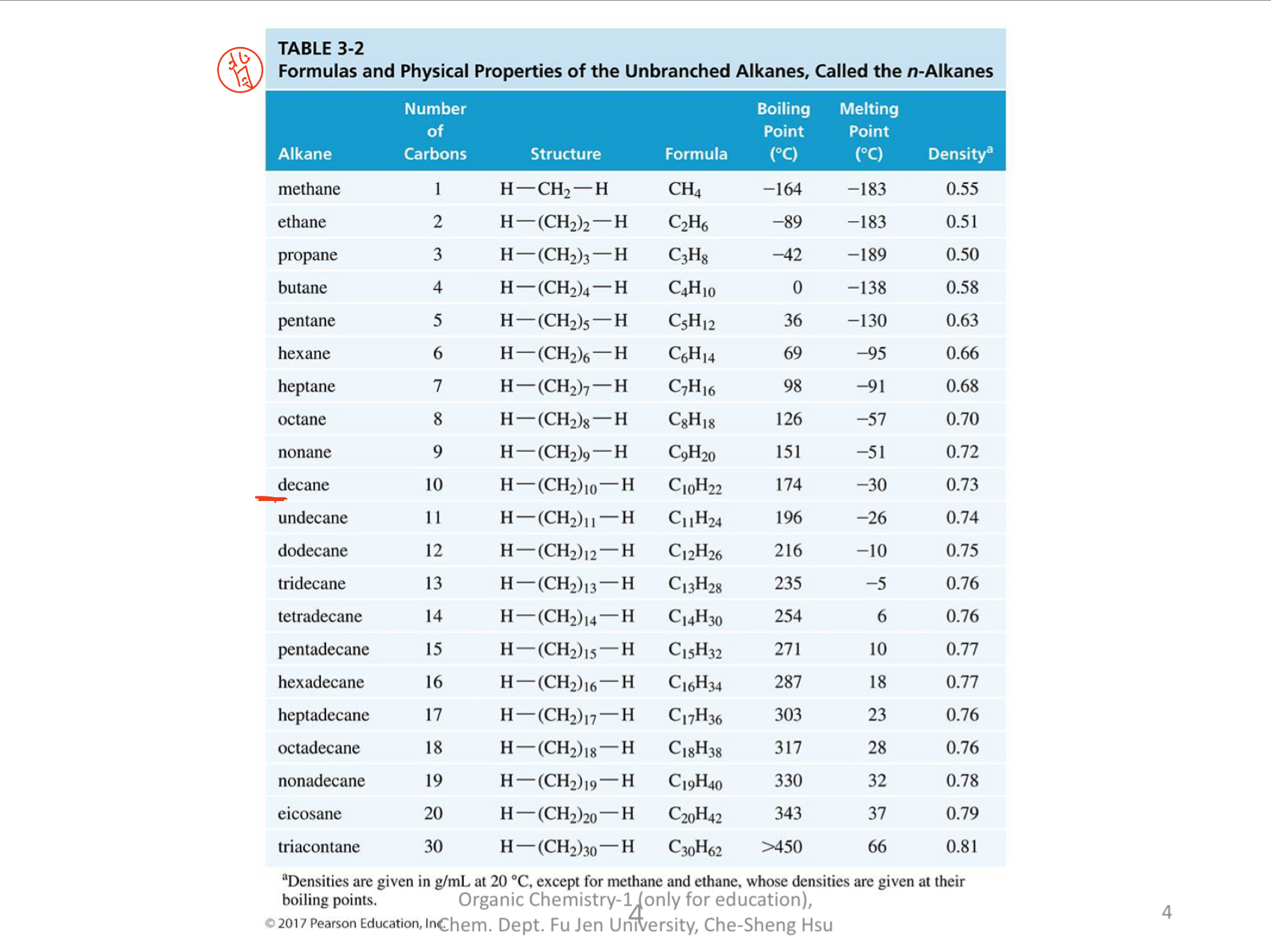
What are the boiling points, melting points, and densities of the unbranched alkanes listed in the table?
Understand the Problem
The question is about the formulas and physical properties of unbranched alkanes, specifically looking for information on their boiling points, melting points, and densities as detailed in a provided table. It seems focused on understanding the characteristics of various alkanes listed, such as decane, undecane, etc.
Answer
The table contains the boiling points, melting points, and densities of the unbranched alkanes.
The boiling points, melting points, and densities of the unbranched alkanes are listed in the table.
Answer for screen readers
The boiling points, melting points, and densities of the unbranched alkanes are listed in the table.
More Information
The properties of n-alkanes vary systematically with increasing molecular weight and chain length. Generally, as the number of carbon atoms increases, both the melting and boiling points increase. Similarly, density tends to increase with molecular weight.
Tips
Common mistakes include confusing boiling points with melting points and overlooking the systematic trends in properties as alkane chain length increases.
Sources
- 3.5: Properties of Alkanes - Chemistry LibreTexts - chem.libretexts.org
- 4.2: Physical Properties of Alkanes - Chemistry LibreTexts - chem.libretexts.org
- Alkane - Wikipedia - en.wikipedia.org
AI-generated content may contain errors. Please verify critical information