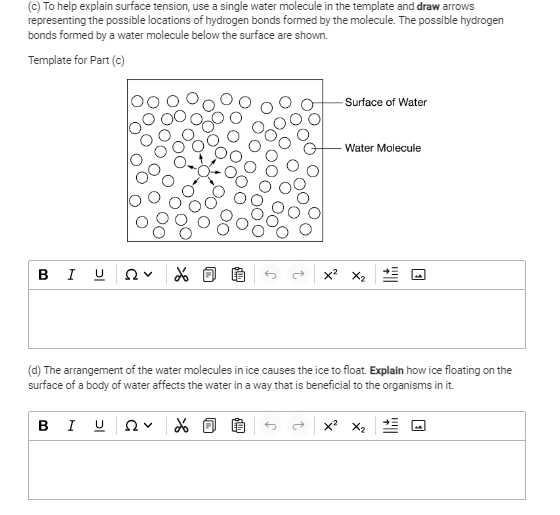
To help explain surface tension, use a single water molecule in the template and draw arrows representing the possible locations of hydrogen bonds formed by the molecule. Explain h... To help explain surface tension, use a single water molecule in the template and draw arrows representing the possible locations of hydrogen bonds formed by the molecule. Explain how ice floating on the surface of a body of water affects the water in a way that is beneficial to the organisms in it.
Understand the Problem
The question is asking for an explanation of surface tension using a diagram of water molecules and requires an understanding of how ice's structure allows it to float, affecting aquatic organisms positively.
Answer
Ice floats due to a less dense structure, insulating and protecting aquatic life.
Water molecules can form up to four hydrogen bonds with adjacent molecules. As water cools to form ice, the hydrogen bonds stabilize, forming a structure that is less dense than liquid water, causing ice to float. This insulating layer of ice helps maintain a stable environment, preventing the water below from freezing solid and allowing aquatic organisms to survive.
Answer for screen readers
Water molecules can form up to four hydrogen bonds with adjacent molecules. As water cools to form ice, the hydrogen bonds stabilize, forming a structure that is less dense than liquid water, causing ice to float. This insulating layer of ice helps maintain a stable environment, preventing the water below from freezing solid and allowing aquatic organisms to survive.
More Information
The crystalline lattice of ice is less dense than liquid water due to more space between molecules. This property is crucial for the survival of many aquatic species during cold periods.
Tips
Mistakenly thinking ice is denser than water is common. Remember, the spacing in ice's crystalline lattice is greater.
Sources
- Surface tension (video) | Chemistry of life - Khan Academy - khanacademy.org
- Properties of Water FRQ Analysis.pdf - Course Hero - coursehero.com
AI-generated content may contain errors. Please verify critical information