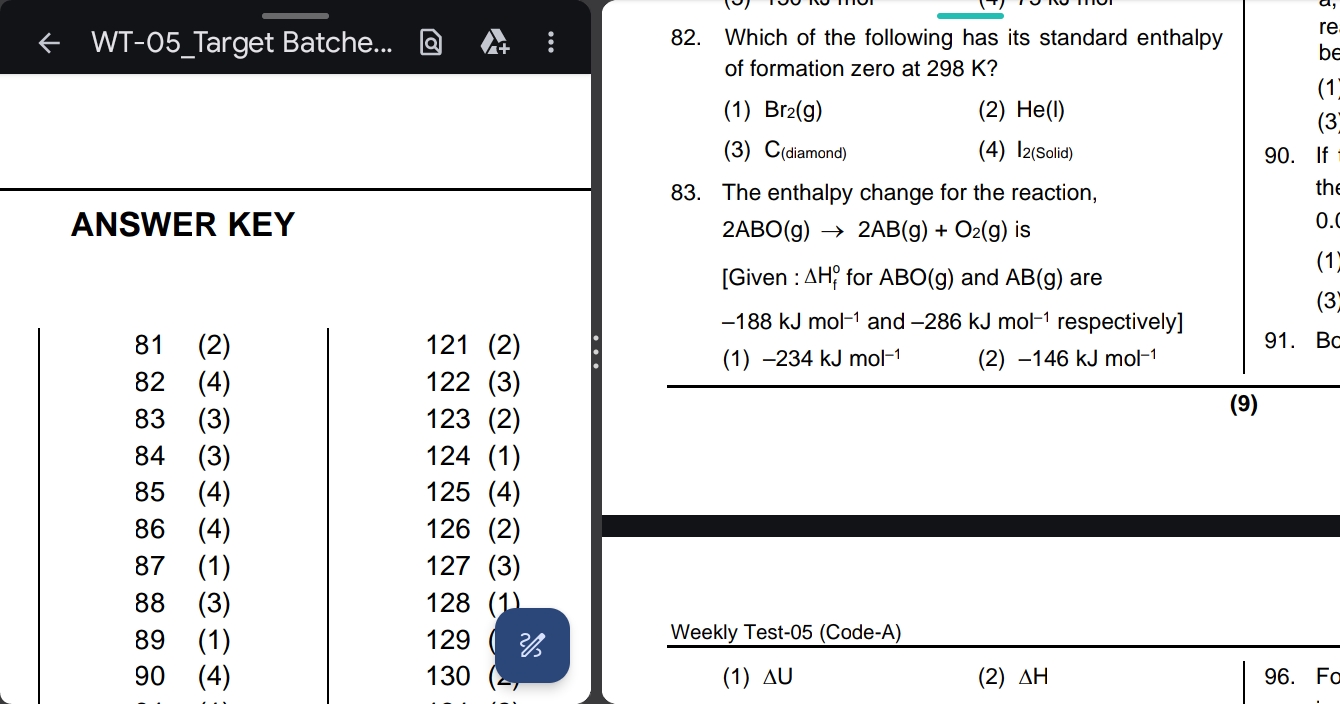
The enthalpy change for the reaction 2ABO(g) → 2AB(g) + O2(g) is given that ΔH°f for ABO(g) is -188 kJ mol-1 and for AB(g) is -286 kJ mol-1 respectively. What is the enthalpy chang... The enthalpy change for the reaction 2ABO(g) → 2AB(g) + O2(g) is given that ΔH°f for ABO(g) is -188 kJ mol-1 and for AB(g) is -286 kJ mol-1 respectively. What is the enthalpy change?
Understand the Problem
The question is asking about the enthalpy change for the reaction 2ABO(g) → 2AB(g) + O2(g) using provided standard enthalpy of formation values. The user needs to calculate the enthalpy change based on the given information.
Answer
The enthalpy change for the reaction is \( -196 \, \text{kJ} \).
Answer for screen readers
The enthalpy change for the reaction is ( -196 , \text{kJ} ).
Steps to Solve
- Identify the reaction and enthalpy values
We start with the reaction:
$$ 2ABO(g) \rightarrow 2AB(g) + O_2(g) $$
The given standard enthalpy of formation values are:
- For ( ABO(g) ): ( \Delta H_f^0 = -188 , \text{kJ mol}^{-1} )
- For ( AB(g) ): ( \Delta H_f^0 = -286 , \text{kJ mol}^{-1} )
- The standard enthalpy of formation for ( O_2(g) ) is zero because it is an elemental form.
- Calculate the total enthalpy of products
To calculate the total enthalpy of the products:
- The reaction produces 2 moles of ( AB(g) ) and 1 mole of ( O_2(g) ).
The total enthalpy for the products:
$$ \Delta H_{products} = 2 \times \Delta H_f^0(AB) + 1 \times \Delta H_f^0(O_2) $$
Substituting the values:
$$ \Delta H_{products} = 2 \times (-286 , \text{kJ mol}^{-1}) + 1 \times (0 , \text{kJ mol}^{-1}) $$
$$ \Delta H_{products} = -572 , \text{kJ} $$
- Calculate the total enthalpy of reactants
Next, calculate the total enthalpy for the reactants:
- The reaction has 2 moles of ( ABO(g) ).
The total enthalpy for the reactants:
$$ \Delta H_{reactants} = 2 \times \Delta H_f^0(ABO) $$
Substituting in the value:
$$ \Delta H_{reactants} = 2 \times (-188 , \text{kJ mol}^{-1}) $$
$$ \Delta H_{reactants} = -376 , \text{kJ} $$
- Calculate the enthalpy change
Now, we can find the enthalpy change for the reaction using:
$$ \Delta H_{reaction} = \Delta H_{products} - \Delta H_{reactants} $$
Substituting the calculated values:
$$ \Delta H_{reaction} = (-572 , \text{kJ}) - (-376 , \text{kJ}) $$
$$ \Delta H_{reaction} = -572 + 376 = -196 , \text{kJ} $$
The enthalpy change for the reaction is ( -196 , \text{kJ} ).
More Information
The enthalpy change represents the heat absorbed or released during the reaction. A negative value indicates that the reaction is exothermic, meaning it releases heat.
Tips
- Forgetting to account for the coefficients in the balanced chemical reaction when calculating the enthalpy for the products and reactants.
- Not recognizing that the standard enthalpy of formation for an element in its most stable form is zero.