Sulphur trioxide is prepared by the following two reactions: S8(s) + 8O2(g) → 8SO2(g) and 2SO2(g) + O2(g) → 2SO3(g).
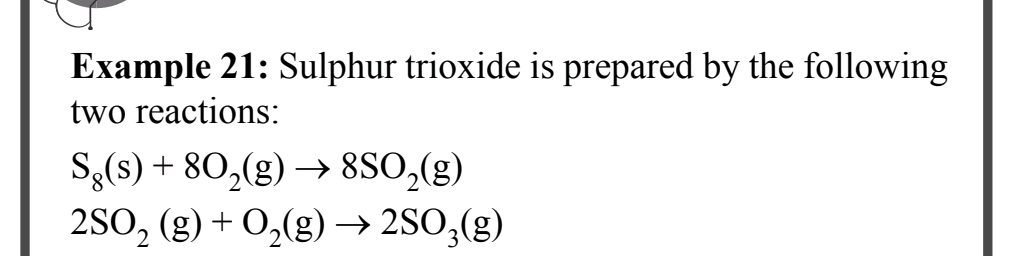
Understand the Problem
The question presents two chemical reactions related to the preparation of sulfur trioxide (SO3) from sulfur (S8) and oxygen (O2). It seems aimed at understanding chemical reactions and stoichiometry.
Answer
640.48 grams of SO3 are produced from 1 mole of S8.
The final answer is 640.48 grams of SO3 are produced from 1 mole of S8.
Answer for screen readers
The final answer is 640.48 grams of SO3 are produced from 1 mole of S8.
More Information
The complete conversion of SO2 to SO3 under these conditions results in the formation of 640.48 grams of SO3 from one mole of S8.
Tips
Ensure correct molar mass calculations and use stoichiometry accurately in following the reactions.
Sources
AI-generated content may contain errors. Please verify critical information